
What is Hoffmann elimination?
Answer
590.1k+ views
Hint: The reaction is named after the name of a scientist, August Wilhelm von Hoffmann, who discovered this reaction. This elimination reaction starts with the treatment of the amine with excess methyl iodide to form ammonium salt. Then, silver oxide and water to form hydroxide of ammonia. The salt is decomposed by heat, to give the Hofmann product.
Complete step by step answer:
Let us discuss this reaction through its mechanism:
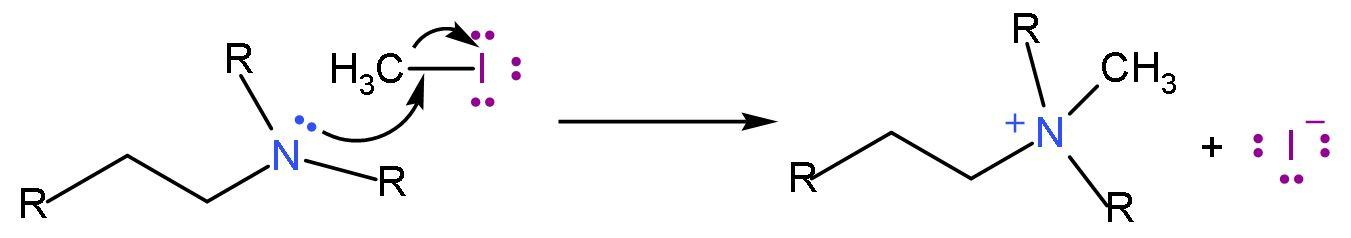
Step (1)- Amine is treated with excess methyl iodide to form the quaternary ammonium iodide salt.
Step (2)- When, iodide ion reacts with silver oxide, the substitution of the iodide ion with the hydroxide ion occurs and also the successive deprotonation of water by the silver oxide ion. The reaction is

Step (3)- The mixture is heated to form the required product, which is tertiary amine and alkene. It is an elimination reaction. The least substituted alkene or the Hofmann product is formed due to the steric hindrance of the leaving group. This is known as the Hoffmann alkene synthesis rule. The last step is depicted as

Note: The Hofmann product is opposite to the product formed by Zaitsev method. This method states that ‘the major alkene formed is the one that corresponds to removal of the hydrogen from the alpha-carbon having the lesser hydrogen atoms’. Hoffmann eliminated products are less stable according to Zaitsev product.
Complete step by step answer:
Let us discuss this reaction through its mechanism:
Step (1)- Amine is treated with excess methyl iodide to form the quaternary ammonium iodide salt.

Step (2)- When, iodide ion reacts with silver oxide, the substitution of the iodide ion with the hydroxide ion occurs and also the successive deprotonation of water by the silver oxide ion. The reaction is

Step (3)- The mixture is heated to form the required product, which is tertiary amine and alkene. It is an elimination reaction. The least substituted alkene or the Hofmann product is formed due to the steric hindrance of the leaving group. This is known as the Hoffmann alkene synthesis rule. The last step is depicted as

Note: The Hofmann product is opposite to the product formed by Zaitsev method. This method states that ‘the major alkene formed is the one that corresponds to removal of the hydrogen from the alpha-carbon having the lesser hydrogen atoms’. Hoffmann eliminated products are less stable according to Zaitsev product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




