
What is Landolt’s experiment?
Answer
505.8k+ views
Hint: Landolt’s experiment is used to define the “law of conservation of mass”. Law of conservation of mass states that mass can neither be created nor be destroyed in chemical or physical reaction or transformation. So we will first understand what this law is all about and how Landolt’s experiment defines or justifies it.
Complete answer:
We know that the law of conservation of mass states that mass can neither be created nor be destroyed in chemical or physical reaction or transformation. It can also be defined as during a chemical or physical reaction, the mass of the reactants consumed must be equal to the mass of products formed in that reaction.
The experimental setup of Landolt’s experiment is:
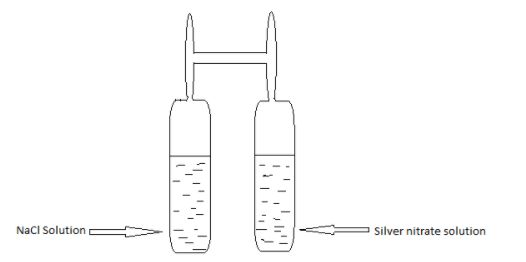
Some main features of Landolt’s experiment are:
In this experiment, a H-shaped tube which is especially designed for this process is taken. In one limb of the tube Silver nitrate solution $(AgN{O_3})$ was taken and in the other limb sodium chloride solution $NaCl$ was taken. And when the tube was sealed, its weight was measured.
Then, they were made to react with each other and after the reaction, the sealed tube’s weight was measured again. And it was found that the weight of the tube before and after the reaction was the same.
Hence, this law defines and justifies the Law of conservation of mass.
Note:
Law of conservation of mass has many applications in the industrial field as it helps to calculate the number of moles (amount) of reactant consumed in a chemical reaction and the number of moles (amount) of product formed during a chemical reaction or the physical reaction or transformation.
Complete answer:
We know that the law of conservation of mass states that mass can neither be created nor be destroyed in chemical or physical reaction or transformation. It can also be defined as during a chemical or physical reaction, the mass of the reactants consumed must be equal to the mass of products formed in that reaction.
The experimental setup of Landolt’s experiment is:

Some main features of Landolt’s experiment are:
In this experiment, a H-shaped tube which is especially designed for this process is taken. In one limb of the tube Silver nitrate solution $(AgN{O_3})$ was taken and in the other limb sodium chloride solution $NaCl$ was taken. And when the tube was sealed, its weight was measured.
Then, they were made to react with each other and after the reaction, the sealed tube’s weight was measured again. And it was found that the weight of the tube before and after the reaction was the same.
Hence, this law defines and justifies the Law of conservation of mass.
Note:
Law of conservation of mass has many applications in the industrial field as it helps to calculate the number of moles (amount) of reactant consumed in a chemical reaction and the number of moles (amount) of product formed during a chemical reaction or the physical reaction or transformation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




