
What is meant by galvanization?
Answer
573.6k+ views
Hint: The process of applying a thin layer of zinc or coat zinc on the thicker base metal is known as the galvanization process. The process resists the corrosion of metal. Galvanisation is done by dipping a metal to be protected in hot and molten zinc by the process of electroplating. The galvanization process is carried out in presence of an electric charge. This is known as electrogalvanization.
Complete answer:
Galvanisation process is used for the protection of iron and steel from corrosion. This zinc coated surface prevents the interaction of the surface with the atmosphere and thus avoids corrosion and weathering effects. Below are the steps involved in the galvanization process. Galvanisation involves three basic steps. These are:
1) Surface preparation
2) Fluxing
3) Galvanising
The first step involves the preparation of the surface of the metal. It is a chemical treatment to remove any grease, dirt from the surface. This prevents the surface from etching. The fluxing process is used to check the wettability of the surface, its chemical reaction between zinc and iron during the immersion, and prevents the further oxidation of iron before immersing in the zinc bath. Final step i.e.galvanisation involves the immersion of metal in a hot zinc bath. The time of immersion depends on the type of metal and the thickness of the coat. Metal is immersed in the molten bath at about 50 degrees. During the immersion molten zinc reacts with the metal. The general reaction to galvanization is as shown below,
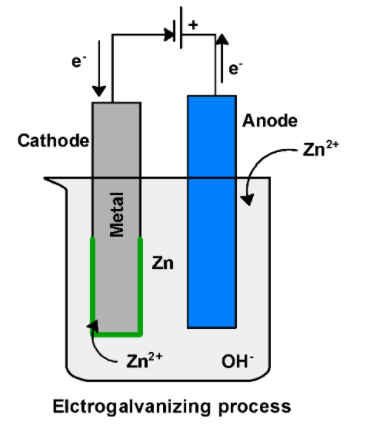
The galvanization process can be carried by the electric charge. Cathode is a metal which needs to be galvanized. These electrodes are connected to the external electricity source .Electrodes are dipped in the zinc metal bath. The bath contains the conducting set such as caustic soda to boost the conductivity. Zinc dissolved in the bath reduced at the anode $\text{ Z}{{\text{n}}^{\text{2+}}}\text{ }$ and deposited on the metal surface. In the galvanization process, zinc ion $\text{ Z}{{\text{n}}^{\text{2+}}}\text{ }$ accepts the electrons from the cathode surface and deposited on the cathode.
$\text{ Z}{{\text{n}}^{\text{2+}}}\text{ }+2{{e}^{-}}\text{ }\to \text{ Zn (deposited on metal) }$
Through the electrogalvanization, the metal surface is coated with zinc metal and thus reduces the corrosion of metal.
Note: Note that galvanization has some disadvantages.
1) Galvanization process only provides the surface from the corrosion for a certain period as the zinc layer is consumed in the process
2) Zinc galvanization is not very useful in highly corrosive areas.
Complete answer:
Galvanisation process is used for the protection of iron and steel from corrosion. This zinc coated surface prevents the interaction of the surface with the atmosphere and thus avoids corrosion and weathering effects. Below are the steps involved in the galvanization process. Galvanisation involves three basic steps. These are:
1) Surface preparation
2) Fluxing
3) Galvanising
The first step involves the preparation of the surface of the metal. It is a chemical treatment to remove any grease, dirt from the surface. This prevents the surface from etching. The fluxing process is used to check the wettability of the surface, its chemical reaction between zinc and iron during the immersion, and prevents the further oxidation of iron before immersing in the zinc bath. Final step i.e.galvanisation involves the immersion of metal in a hot zinc bath. The time of immersion depends on the type of metal and the thickness of the coat. Metal is immersed in the molten bath at about 50 degrees. During the immersion molten zinc reacts with the metal. The general reaction to galvanization is as shown below,

The galvanization process can be carried by the electric charge. Cathode is a metal which needs to be galvanized. These electrodes are connected to the external electricity source .Electrodes are dipped in the zinc metal bath. The bath contains the conducting set such as caustic soda to boost the conductivity. Zinc dissolved in the bath reduced at the anode $\text{ Z}{{\text{n}}^{\text{2+}}}\text{ }$ and deposited on the metal surface. In the galvanization process, zinc ion $\text{ Z}{{\text{n}}^{\text{2+}}}\text{ }$ accepts the electrons from the cathode surface and deposited on the cathode.
$\text{ Z}{{\text{n}}^{\text{2+}}}\text{ }+2{{e}^{-}}\text{ }\to \text{ Zn (deposited on metal) }$
Through the electrogalvanization, the metal surface is coated with zinc metal and thus reduces the corrosion of metal.
Note: Note that galvanization has some disadvantages.
1) Galvanization process only provides the surface from the corrosion for a certain period as the zinc layer is consumed in the process
2) Zinc galvanization is not very useful in highly corrosive areas.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




