
What is Meta Directing Effect?
Answer
535.5k+ views
Hint: When a compound is present on the benzene ring is a deactivating group then the incoming electrophile or nucleophile attaches to the Meta position and if the compound present on the benzene ring is an activating group then the incoming electrophile or nucleophile attaches to the ortho or para positions.
Complete step-by-step answer:
To understand the Meta Directing Effect, let us understand the activating and deactivating groups.
When the electrons are donated to the benzene ring, then the benzene will be activated and the rate of the reaction will be increased. If the incoming electrophile or nucleophile is attached to the ortho, or para positions then the compound will be an activating group.
When the electrons are taken from the benzene ring, then the benzene will be deactivated and the rate of the reaction will be decreased. If the incoming electrophile or nucleophile is attached to the Meta position then the compound will be a deactivating group.
So, when the deactivating group present on the benzene ring then it will take the electrons from the ortho and para positions, which will create an increase in electron density on the meta position, and the incoming compound will attack the meta-position, and this is known as Meta Directing effect. An example, when Nitrobenzene is treated with methyl chloride, then the methyl group attaches to the meta-position because the nitro group is a deactivating group. The reaction is given below:
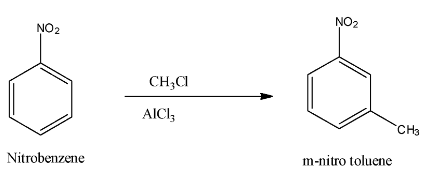
Note: But when the toluene is treated with nitric acid, the nitro group attaches to the ortho or para position because methyl is an activating group. The reaction is given below:
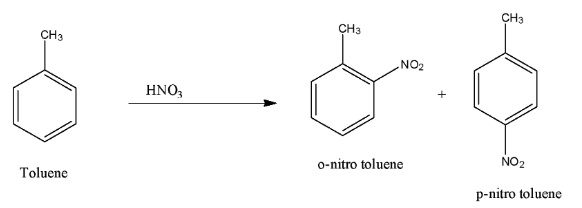
Complete step-by-step answer:
To understand the Meta Directing Effect, let us understand the activating and deactivating groups.
When the electrons are donated to the benzene ring, then the benzene will be activated and the rate of the reaction will be increased. If the incoming electrophile or nucleophile is attached to the ortho, or para positions then the compound will be an activating group.
When the electrons are taken from the benzene ring, then the benzene will be deactivated and the rate of the reaction will be decreased. If the incoming electrophile or nucleophile is attached to the Meta position then the compound will be a deactivating group.
So, when the deactivating group present on the benzene ring then it will take the electrons from the ortho and para positions, which will create an increase in electron density on the meta position, and the incoming compound will attack the meta-position, and this is known as Meta Directing effect. An example, when Nitrobenzene is treated with methyl chloride, then the methyl group attaches to the meta-position because the nitro group is a deactivating group. The reaction is given below:

Note: But when the toluene is treated with nitric acid, the nitro group attaches to the ortho or para position because methyl is an activating group. The reaction is given below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




