
What is resonance hybrid?
Answer
531k+ views
Hint :We know that the resonance is a representation of movement of pi electron clouds in a molecule having alternate double bonds. All the resonating structures produced by a molecule have equal contribution to the resonance hybrid.
Complete Step By Step Answer:
We know that resonance is a theoretical way used for describing the bonding between certain atoms in a molecule or ions with the help of combination of a number of contributing structures or forms which are generally known as resonating structures, or canonical structures or kekule’s structure.
We know that when a structure undergoes the process of resonance the electron clouds in the double bond gets delocalized throughout the bonds. Let’s take here an example of benzene which is a resonance hybrid.
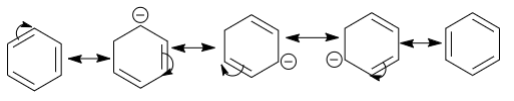
Now if we consider the structures we can see that the electron cloud jumps or gets delocalized in all the carbons one by one giving rise to corresponding resonating structures. All these structures exist theoretically in nature and the kekule’s structure is the final structure which we can observe. It represents the resonance hybrids, which is the sum of all these structures as all of these structures contribute to the final structure of the benzene. All the bonds involved in resonance have equal lengths.
Therefore, the benzene acquires kekule structures back and forth, again, we know that there is no fixed calculated structure and we cannot say that the benzene changes its electron clouds back and forth, as there is no fixed rule as to which structure it will acquire and when, the kekule structures just gives us an idea that the electrons clouds are delocalized.
Note :
Resonance is a theoretical effect to get an idea about the movement of electron clouds in the double bonds, and consequently a prediction of the preferable positions of nucleophiles or electrophiles which will attack the original structure.
Complete Step By Step Answer:
We know that resonance is a theoretical way used for describing the bonding between certain atoms in a molecule or ions with the help of combination of a number of contributing structures or forms which are generally known as resonating structures, or canonical structures or kekule’s structure.
We know that when a structure undergoes the process of resonance the electron clouds in the double bond gets delocalized throughout the bonds. Let’s take here an example of benzene which is a resonance hybrid.

Now if we consider the structures we can see that the electron cloud jumps or gets delocalized in all the carbons one by one giving rise to corresponding resonating structures. All these structures exist theoretically in nature and the kekule’s structure is the final structure which we can observe. It represents the resonance hybrids, which is the sum of all these structures as all of these structures contribute to the final structure of the benzene. All the bonds involved in resonance have equal lengths.
Therefore, the benzene acquires kekule structures back and forth, again, we know that there is no fixed calculated structure and we cannot say that the benzene changes its electron clouds back and forth, as there is no fixed rule as to which structure it will acquire and when, the kekule structures just gives us an idea that the electrons clouds are delocalized.
Note :
Resonance is a theoretical effect to get an idea about the movement of electron clouds in the double bonds, and consequently a prediction of the preferable positions of nucleophiles or electrophiles which will attack the original structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




