
What is Rutherford’s atomic model?
Answer
478.2k+ views
Hint: In this question we have been asked about Rutherford's atomic model. Therefore, to answer this question we shall discuss Rutherford's atomic model. We shall also discuss the famous gold foil experiment by Rutherford that helped him obtain the atomic model.
Complete step by step answer:
After the discovery of atoms by John Dalton and electrons by J.J Thompson, Rutherford discovered the nucleus of atoms. Dalton proposed that atoms were indivisible. However, he was proven wrong after the discovery of the first subatomic particle i.e. the electron. J.J Thompson in his theory proposed that an atom is like a plum pudding, where the negatively charged electrons are scattered in the field of positive charge.
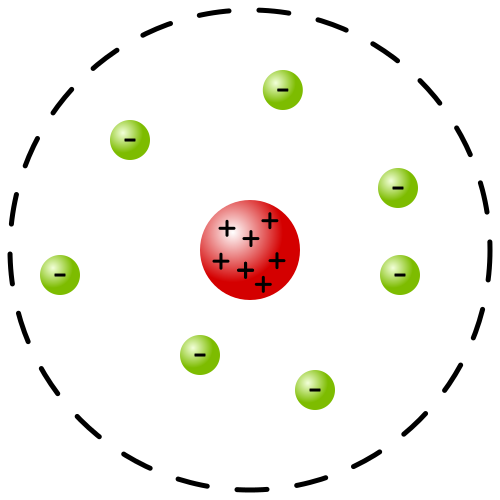
In 1911 Ernest Rutherford carried out an experiment through which he confirmed that an atom is made of a massive region of positive charge at the centre and electrons revolving around this positive charge. In his experiment of gold foil, Rutherford bombarded the alpha particles on a very thin sheet of gold foil. Surrounding this foil, he placed a photosensitive screen that would light up as the alpha particles struck it. When he saw the results, he observed that all the positive charge was concentrated at the centre of the nucleus as shown in the above diagram. The experiment showed that the rest of the atom is empty except for electrons which revolve around the nucleus. Rutherford’s model of atoms represented the solar system. Where the positive charge is at the centre like the sun and electrons revolve around it like a planet.
Therefore, his model is known as the planetary model.
Note: Rutherford also predicted the presence of the neutral particles known as neutrons. However, he was unable to find the neutrons through experiments. Several years later, the neutrons were discovered by his student James Chadwick. Even though new models of atoms have been put forward by other scientists such as Niels Bohr, Rutherford's atomic model is still used on a primary level to explain the structure of atoms. It is, however, not very popular with higher level education.
Complete step by step answer:
After the discovery of atoms by John Dalton and electrons by J.J Thompson, Rutherford discovered the nucleus of atoms. Dalton proposed that atoms were indivisible. However, he was proven wrong after the discovery of the first subatomic particle i.e. the electron. J.J Thompson in his theory proposed that an atom is like a plum pudding, where the negatively charged electrons are scattered in the field of positive charge.

In 1911 Ernest Rutherford carried out an experiment through which he confirmed that an atom is made of a massive region of positive charge at the centre and electrons revolving around this positive charge. In his experiment of gold foil, Rutherford bombarded the alpha particles on a very thin sheet of gold foil. Surrounding this foil, he placed a photosensitive screen that would light up as the alpha particles struck it. When he saw the results, he observed that all the positive charge was concentrated at the centre of the nucleus as shown in the above diagram. The experiment showed that the rest of the atom is empty except for electrons which revolve around the nucleus. Rutherford’s model of atoms represented the solar system. Where the positive charge is at the centre like the sun and electrons revolve around it like a planet.
Therefore, his model is known as the planetary model.
Note: Rutherford also predicted the presence of the neutral particles known as neutrons. However, he was unable to find the neutrons through experiments. Several years later, the neutrons were discovered by his student James Chadwick. Even though new models of atoms have been put forward by other scientists such as Niels Bohr, Rutherford's atomic model is still used on a primary level to explain the structure of atoms. It is, however, not very popular with higher level education.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




