
What is \[SS\] bond?
Answer
507k+ views
Hint: We know that \[S\] stands for sulphur in the periodic table. So \[SS\] bond is a bond between two sulphur atoms. We have a lot of sulphur containing compounds that have these kinds of \[SS\] bonds.
These are usually present as a functional groups in most of the molecules with the structure \[R - S - S - R'\]
Complete answer:
In chemistry we have learned a lot about different kinds of bonds between different types of atoms.
One of the important kinds of such a bond is called the disulfide bond or disulfide bridge also known as \[SS\] bond. These bonds are usually formed by the coupling of two thiol groups.
Disulfide bonds are usually strong present both in organic and inorganic molecules.
The \[SS\] bond is a covalent bond formed by the sharing of electrons by the two sulphur atoms.
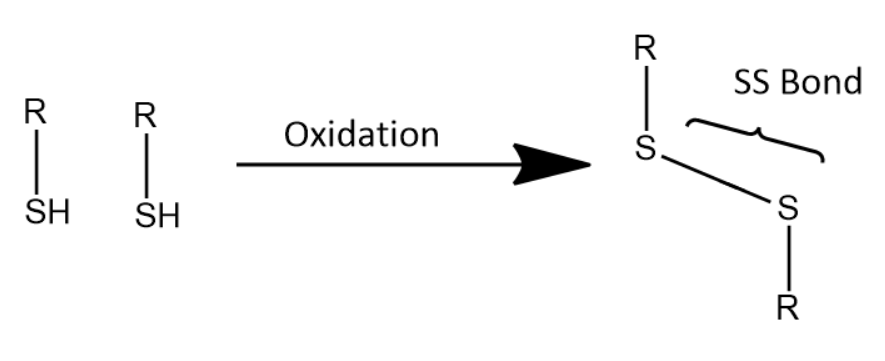
One way of obtaining \[SS\] bond is by the following reaction:
Additional Information:
By using processes such as vulcanization we can introduce disulfide bonds in rubber. This can increase the elastic nature of rubber and also helps to increase properties such as tensile strength, hardness and weather resistance. This is a very important reaction used in the rubber industry.
\[SS\] bonds are also seen in proteins. Some amino acids such as cysteine have sulphur in them. When they form proteins they will undergo disulfide linkage. This serves a lot of functions in the biological systems. This is also a reason for the stability of some proteins in their folded form.
Note:
\[SS\] bond is also found in oxy-sulphur compounds such as \[\;{H_2}{S_2}{O_6}\] (Dithionic acid ) and \[{H_2}{S_2}{O_5}\;\] (Disulfurous acid)
These are usually present as a functional groups in most of the molecules with the structure \[R - S - S - R'\]
Complete answer:
In chemistry we have learned a lot about different kinds of bonds between different types of atoms.
One of the important kinds of such a bond is called the disulfide bond or disulfide bridge also known as \[SS\] bond. These bonds are usually formed by the coupling of two thiol groups.
Disulfide bonds are usually strong present both in organic and inorganic molecules.
The \[SS\] bond is a covalent bond formed by the sharing of electrons by the two sulphur atoms.
One way of obtaining \[SS\] bond is by the following reaction:

Additional Information:
By using processes such as vulcanization we can introduce disulfide bonds in rubber. This can increase the elastic nature of rubber and also helps to increase properties such as tensile strength, hardness and weather resistance. This is a very important reaction used in the rubber industry.
\[SS\] bonds are also seen in proteins. Some amino acids such as cysteine have sulphur in them. When they form proteins they will undergo disulfide linkage. This serves a lot of functions in the biological systems. This is also a reason for the stability of some proteins in their folded form.
Note:
\[SS\] bond is also found in oxy-sulphur compounds such as \[\;{H_2}{S_2}{O_6}\] (Dithionic acid ) and \[{H_2}{S_2}{O_5}\;\] (Disulfurous acid)
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




