
What is the formula of dihydrogen?
Answer
508.2k+ views
Hint: Dihydrogen is a molecule formed from the hydrogen atoms. It is a homonuclear molecule which means it is a molecule which has the same atoms in it and no other atom is part of the molecule. This molecule consists of a covalent bond between the hydrogen atoms. It is a molecule with linear shape because a single bond is present.
Complete answer:
Dihydrogen is a gas which is made up of hydrogen atoms which is the lightest atom and hence, it is the lightest gas. Dihydrogen is a colourless, tasteless, and odourless gas and it is highly combustible in nature.
Dihydrogen as the name suggests consists of two hydrogens atoms in the molecule.
The structure of dihydrogen gas is:
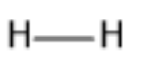
Therefore, we can say that the molecular formula of dihydrogen is ${H_2}$ .
Additional information:
(1) This molecule is nonpolar and has a linear shape.
(2) Each hydrogen atom shares one electron towards the covalent bond.
(3) The boiling point of dihydrogen is $20.271K$ or $ - {252.879^o}C$ .
(4) The latent heat of fusion of dihydrogen molecule is $0.117{\text{KJmo}}{{\text{l}}^{{\text{ - 1}}}}$ .
(5) The latent heat of vaporization (enthalpy of vaporization) of dihydrogen is $0.904KJmo{l^{ - 1}}$.
(6) The molar heat capacity of this molecule is around $28.83J{K^{ - 1}}$ .
Note:
There are many applications of dihydrogen gas such as it is used in manufacturing of ammonia and for refining some of the fossil fuels. Dihydrogen is a good reducing agent and hence can be used in many reactions. It can be used as a hydrogenating agent to increase the saturation level of the unsaturated oils and fats.
Complete answer:
Dihydrogen is a gas which is made up of hydrogen atoms which is the lightest atom and hence, it is the lightest gas. Dihydrogen is a colourless, tasteless, and odourless gas and it is highly combustible in nature.
Dihydrogen as the name suggests consists of two hydrogens atoms in the molecule.
The structure of dihydrogen gas is:
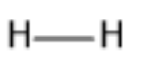
Therefore, we can say that the molecular formula of dihydrogen is ${H_2}$ .
Additional information:
(1) This molecule is nonpolar and has a linear shape.
(2) Each hydrogen atom shares one electron towards the covalent bond.
(3) The boiling point of dihydrogen is $20.271K$ or $ - {252.879^o}C$ .
(4) The latent heat of fusion of dihydrogen molecule is $0.117{\text{KJmo}}{{\text{l}}^{{\text{ - 1}}}}$ .
(5) The latent heat of vaporization (enthalpy of vaporization) of dihydrogen is $0.904KJmo{l^{ - 1}}$.
(6) The molar heat capacity of this molecule is around $28.83J{K^{ - 1}}$ .
Note:
There are many applications of dihydrogen gas such as it is used in manufacturing of ammonia and for refining some of the fossil fuels. Dihydrogen is a good reducing agent and hence can be used in many reactions. It can be used as a hydrogenating agent to increase the saturation level of the unsaturated oils and fats.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




