
What is the Lewis structure for $Ge{{F}_{4}}$?
Answer
527.1k+ views
Hint: To solve this question we first need to know what Lewis dot structures are. The valence electrons of the atoms of a molecule can be represented using the Lewis dot structure. They are a simplified version of the molecular geometry of a compound and can be used to depict the chemical bonding in the molecules.
Complete answer:
The Lewis dot structure of a compound can be drawn by following steps.
1. We first need to know the valence electrons in the atoms of the molecule.
The atomic number of germanium (Ge) is 32 and has 4 valence electrons.
Whereas the atomic number of fluorine (F) is 9 and has 7 valence electrons.
2. Identify the least electronegative atom. This atom is the central atom of the molecule.
Germanium is the least electronegative atom in $Ge{{F}_{4}}$.
3. Connect the atoms via single bonds to form a skeleton structure.
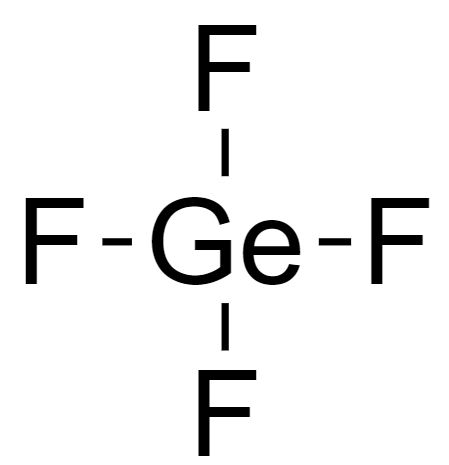
4. Assign lone pairs to each molecule.
Since a Ge atom is connected to four F atoms, it has no lone pairs.
Whereas each F atom is bonded to one Ge atom, so one electron of the valence electron is used during bonding, and hence the number of lone pairs on F is 3.
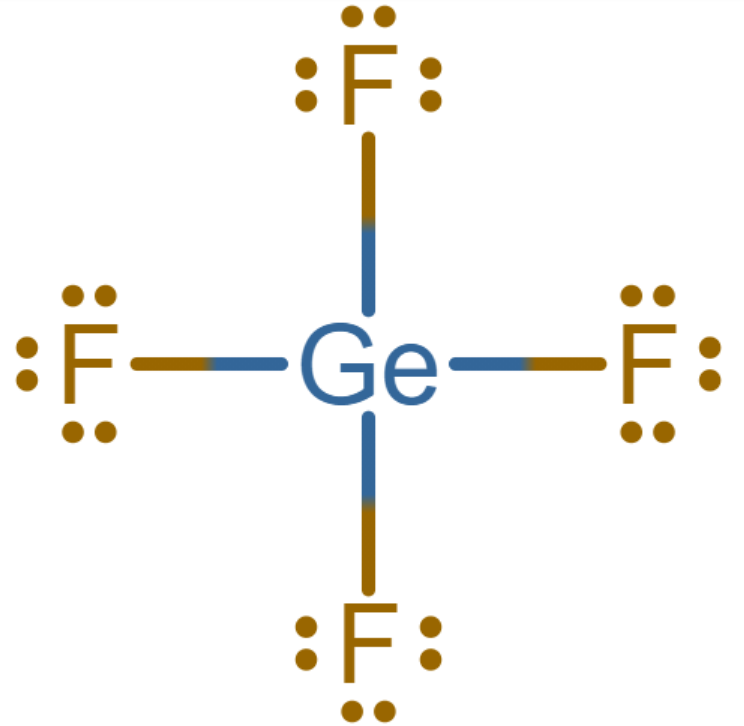
5. Check if every atom has an octet configuration. Draw double or triple bonds to satisfy the octet.
Since the octet is satisfied for both germanium and fluorine, the structure drawn above is the Lewis dot structure of $Ge{{F}_{4}}$.
Note:
It should be noted that when the molecule is an ion, the electrons added to or subtracted from the Lewis dot structure are equal to the magnitude of the charge. In the case of cation (positively charged ion) electrons are subtracted, and in the case of an anion (negatively charged ions), electrons are added.
Complete answer:
The Lewis dot structure of a compound can be drawn by following steps.
1. We first need to know the valence electrons in the atoms of the molecule.
The atomic number of germanium (Ge) is 32 and has 4 valence electrons.
Whereas the atomic number of fluorine (F) is 9 and has 7 valence electrons.
2. Identify the least electronegative atom. This atom is the central atom of the molecule.
Germanium is the least electronegative atom in $Ge{{F}_{4}}$.
3. Connect the atoms via single bonds to form a skeleton structure.
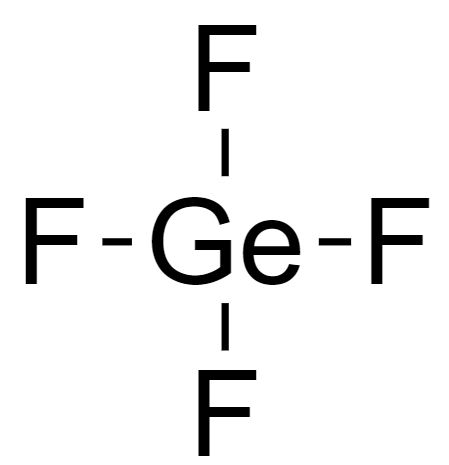
4. Assign lone pairs to each molecule.
Since a Ge atom is connected to four F atoms, it has no lone pairs.
Whereas each F atom is bonded to one Ge atom, so one electron of the valence electron is used during bonding, and hence the number of lone pairs on F is 3.

5. Check if every atom has an octet configuration. Draw double or triple bonds to satisfy the octet.
Since the octet is satisfied for both germanium and fluorine, the structure drawn above is the Lewis dot structure of $Ge{{F}_{4}}$.
Note:
It should be noted that when the molecule is an ion, the electrons added to or subtracted from the Lewis dot structure are equal to the magnitude of the charge. In the case of cation (positively charged ion) electrons are subtracted, and in the case of an anion (negatively charged ions), electrons are added.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




