
What is the molecular shape of $ {{C}_{2}}C{{l}_{2}} $ ?
Answer
493.8k+ views
Hint: The molecular name for $ {{C}_{2}}C{{l}_{2}} $ is Dichloroacetylene. The hydrogen atoms of Acetylene or Ethyne are substituted by two chlorine atoms. As it is a substituted acetylene compound it will have a molecular shape similar to Acetylene.
Complete Step By Step Answer:
The structure of Dichloroacetylene is given below:
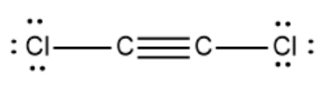
There is a triple bond between the two carbon atoms and the chlorine and carbon atoms are linked by a single bond. The chlorine atom has six valence electrons on it. it shares one electron each with a carbon atom, and carbon shares its one electron to form a covalent bond.
Each carbon atom contributes 4 valence electrons and each chlorine atom gives 7 valence electrons. So, $ {{C}_{2}}C{{l}_{2}} $ the molecule has a total of 22 valence electrons in its Lewis structure.
The triple bond between carbon atoms accounts for 2 electrons each for one single bond so a total of 6 electrons. Thus the two C-Cl bonds give a total of 4 atoms. The chlorine atom has 3 lone pairs of electrons. So it accounts for 6 electrons each from both the atoms which sum up to 12 atoms. This way the electrons $ {{C}_{2}}C{{l}_{2}} $ are 22.
The coordination number of carbon in $ {{C}_{2}}C{{l}_{2}} $ is 2 as two chlorine atoms are attached to the carbon atoms. Also, we know that the molecular shape of acetylene is linear and the bond angle is $ {{180}^{o}} $ . Therefore, according to Valence Shell electron repulsion theory (VSEPR), the molecular geometry of $ {{C}_{2}}C{{l}_{2}} $ is Linear with a bond angle of $ {{180}^{o}} $ .
Final answer: Molecular shape of $ {{C}_{2}}C{{l}_{2}} $ is Linear.
Note:
Steric number and coordination number is the total number of atoms attached to the central atom in a molecule. The steric number is responsible for determining the molecular geometry of a molecule. It is used in VSEPR theory.
Complete Step By Step Answer:
The structure of Dichloroacetylene is given below:
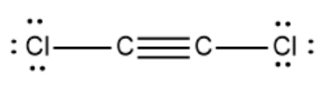
There is a triple bond between the two carbon atoms and the chlorine and carbon atoms are linked by a single bond. The chlorine atom has six valence electrons on it. it shares one electron each with a carbon atom, and carbon shares its one electron to form a covalent bond.
Each carbon atom contributes 4 valence electrons and each chlorine atom gives 7 valence electrons. So, $ {{C}_{2}}C{{l}_{2}} $ the molecule has a total of 22 valence electrons in its Lewis structure.
The triple bond between carbon atoms accounts for 2 electrons each for one single bond so a total of 6 electrons. Thus the two C-Cl bonds give a total of 4 atoms. The chlorine atom has 3 lone pairs of electrons. So it accounts for 6 electrons each from both the atoms which sum up to 12 atoms. This way the electrons $ {{C}_{2}}C{{l}_{2}} $ are 22.
The coordination number of carbon in $ {{C}_{2}}C{{l}_{2}} $ is 2 as two chlorine atoms are attached to the carbon atoms. Also, we know that the molecular shape of acetylene is linear and the bond angle is $ {{180}^{o}} $ . Therefore, according to Valence Shell electron repulsion theory (VSEPR), the molecular geometry of $ {{C}_{2}}C{{l}_{2}} $ is Linear with a bond angle of $ {{180}^{o}} $ .
Final answer: Molecular shape of $ {{C}_{2}}C{{l}_{2}} $ is Linear.
Note:
Steric number and coordination number is the total number of atoms attached to the central atom in a molecule. The steric number is responsible for determining the molecular geometry of a molecule. It is used in VSEPR theory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




