
What is the name of \[HBr\] (in water)?
Answer
491.1k+ views
Hint: Hydrogen bromide comes under the category of inorganic compound which is capable of forming hydrogen bonds due to the presence of electronegative bromine atoms. Hydrogen bromides exist in a gaseous state in the atmosphere.
Complete answer:
Hydrogen bromide is also known as hydrogen halide because halogen atoms are combined with hydrogen atoms to form hydrogen halides. Like other hydrogen halides of the halogen family, hydrogen bromide is a colorless gas which is stable in the atmosphere.
From the structure of hydrogen bromide, it is clear that hydrogen bromide molecules are capable of forming hydrogen bonds. When we dissolve hydrogen bromide in water, it forms hydrogen bonding with oxygen of water to form a solution.
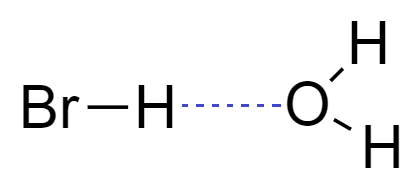
When hydrogen bromide gas dissolves into water to form a solution of Hydrogen bromide it is commonly known as hydrobromic acid.
The chemical reaction of formation of hydrobromic acid from hydrogen bromide is expressed as:
$HB{r_{\left( {s,liq,gas} \right)}} \to HB{r_{\left( {aq} \right)}}$
Aqueous hydrogen bromide is known as acid because after dissolution into water, hydrobromic acid acts as a very strong acid by releasing bromide ion and hydronium ion in the water.
Chemical reaction which shows formation of bromide ion and hydronium ion in water by hydrobromic acid is expressed as:
$HB{r_{\left( {aq} \right)}} + {H_2}O \to {H_3}{O^ + } + B{r^ - }$
Solid gases, or liquid physical state of $HBr$, is known as hydrogen bromide but aqueous $HB{r_{\left( {aq} \right)}}$ is known as hydrobromic acid.
$ \therefore $ The name of \[HBr\] (in water) is Hydrobromic acid.
Note:
Hydrobromic acid forms a saturated solution which contains $68.85\% $ of $HBr$ in it. However, hydrobromic acid that contains $HBr$ by $47.6\% $ of mass forms an azeotropic mixture which shows a boiling point near ${124.3^ \circ }C$.
Complete answer:
Hydrogen bromide is also known as hydrogen halide because halogen atoms are combined with hydrogen atoms to form hydrogen halides. Like other hydrogen halides of the halogen family, hydrogen bromide is a colorless gas which is stable in the atmosphere.
From the structure of hydrogen bromide, it is clear that hydrogen bromide molecules are capable of forming hydrogen bonds. When we dissolve hydrogen bromide in water, it forms hydrogen bonding with oxygen of water to form a solution.

When hydrogen bromide gas dissolves into water to form a solution of Hydrogen bromide it is commonly known as hydrobromic acid.
The chemical reaction of formation of hydrobromic acid from hydrogen bromide is expressed as:
$HB{r_{\left( {s,liq,gas} \right)}} \to HB{r_{\left( {aq} \right)}}$
Aqueous hydrogen bromide is known as acid because after dissolution into water, hydrobromic acid acts as a very strong acid by releasing bromide ion and hydronium ion in the water.
Chemical reaction which shows formation of bromide ion and hydronium ion in water by hydrobromic acid is expressed as:
$HB{r_{\left( {aq} \right)}} + {H_2}O \to {H_3}{O^ + } + B{r^ - }$
Solid gases, or liquid physical state of $HBr$, is known as hydrogen bromide but aqueous $HB{r_{\left( {aq} \right)}}$ is known as hydrobromic acid.
$ \therefore $ The name of \[HBr\] (in water) is Hydrobromic acid.
Note:
Hydrobromic acid forms a saturated solution which contains $68.85\% $ of $HBr$ in it. However, hydrobromic acid that contains $HBr$ by $47.6\% $ of mass forms an azeotropic mixture which shows a boiling point near ${124.3^ \circ }C$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




