
What is the name of $NC{l_3}$ ?
Answer
494.1k+ views
Hint: In the given compound the central metal atom is Nitrogen, which belongs to group XV of the periodic table. There are three chlorine compounds attached to it. We’ll use simple IUPAC nomenclature rules to name the compound.
Complete answer:
Nitrogen has 3 electrons in the outermost shell, it forms three bonds with three Chlorine atoms (Chlorine is from group XVII of the periodic table). If there more than one substituent is attached to the central metal atom, we use the Greek words ``di”, “tri”, “tetra” to determine the no. of substituents attached to it.
The name of the given compound $NC{l_3}$ will be Nitrogen Trichloride. The common name for this compound is Trichloramine. The structure of this compound is as follows:
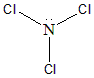
$NC{l_3}$ has $s{p^3}$ hybridisation and tetrahedral geometry, but due to the presence of the lone pair, the lone pair bond pair repulsion reduces the bond angle to ${107^ \circ }$ , to give a Pyramidal Shape.
Nitrogen has one lone pair of electrons. Nitrogen Trichloride is a yellow, oily and pungent smelling compound. It is highly explosive in nature. This compound is responsible for the typical chlorine smell in swimming pools and water tanks.
Additional Information:
The compound is prepared by treatment of ammonium salts with chlorine, primarily ammonium nitrate. The intermediates that are formed in the reaction are monochloramine and dichloramine having chemical formula $N{H_2}Cl\& NHC{l_2}$ respectively.
Note:
Nitrogen Trichloride gives dinitrogen and chlorine gas on explosion. $NC{l_3}$ can be formed in small amounts in swimming pools and water tanks, when they are disinfected with monochloramine and it reacts with urea present in urine and sweat. $NC{l_3}$ is trademarked as Agene and was used to bleach flour, but was later banned for safety concerns.
Complete answer:
Nitrogen has 3 electrons in the outermost shell, it forms three bonds with three Chlorine atoms (Chlorine is from group XVII of the periodic table). If there more than one substituent is attached to the central metal atom, we use the Greek words ``di”, “tri”, “tetra” to determine the no. of substituents attached to it.
The name of the given compound $NC{l_3}$ will be Nitrogen Trichloride. The common name for this compound is Trichloramine. The structure of this compound is as follows:

$NC{l_3}$ has $s{p^3}$ hybridisation and tetrahedral geometry, but due to the presence of the lone pair, the lone pair bond pair repulsion reduces the bond angle to ${107^ \circ }$ , to give a Pyramidal Shape.
Nitrogen has one lone pair of electrons. Nitrogen Trichloride is a yellow, oily and pungent smelling compound. It is highly explosive in nature. This compound is responsible for the typical chlorine smell in swimming pools and water tanks.
Additional Information:
The compound is prepared by treatment of ammonium salts with chlorine, primarily ammonium nitrate. The intermediates that are formed in the reaction are monochloramine and dichloramine having chemical formula $N{H_2}Cl\& NHC{l_2}$ respectively.
Note:
Nitrogen Trichloride gives dinitrogen and chlorine gas on explosion. $NC{l_3}$ can be formed in small amounts in swimming pools and water tanks, when they are disinfected with monochloramine and it reacts with urea present in urine and sweat. $NC{l_3}$ is trademarked as Agene and was used to bleach flour, but was later banned for safety concerns.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




