
What is the octet configuration?
Answer
582.6k+ views
Hint: You can start the solution of this question by describing how the octet rule or octet configuration come into existence and then you can define the octet configuration, followed by the limitations and by giving specific examples to explain it better.
Complete step by step solution:
According to the octet configuration as the name suggests Oct means eight the atom has eight electrons in the outermost orbit.
In the year 1904, Richard Abegg proposed the concept of coordination number and said that the atoms behave as donors or acceptors of electrons. The octet rule uses this idea and states that every atom binds with another atom to have eight electrons in its outermost shell.
The octet rule is able to explain the formation of a number of compounds and also able to explain the stability of the compound. Let’s consider an example of carbon dioxide.
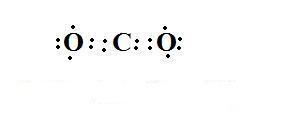
As we can see from the above structure that the carbon “C” and both the oxygen “O” have 8electrons in their outermost shell which make them stable.
Additional information:
Limitations of octet rule:
As hydrogen and helium both have only one and two electrons respectively in their outermost shell it is impossible for them to have eight electrons in their outermost shell. So there are many compounds which have less than eight electrons in their outermost shell but are still stable. Similarly, there are many compounds which have more than eight electrons in their outermost shell but are still stable.
Note: The octet rule is based on the concept that electronic configuration of the noble gases are most stable and they have eight electrons in their outermost orbital so every element should attain the similar electronic configuration to become stable.
Complete step by step solution:
According to the octet configuration as the name suggests Oct means eight the atom has eight electrons in the outermost orbit.
In the year 1904, Richard Abegg proposed the concept of coordination number and said that the atoms behave as donors or acceptors of electrons. The octet rule uses this idea and states that every atom binds with another atom to have eight electrons in its outermost shell.
The octet rule is able to explain the formation of a number of compounds and also able to explain the stability of the compound. Let’s consider an example of carbon dioxide.
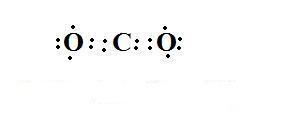
As we can see from the above structure that the carbon “C” and both the oxygen “O” have 8electrons in their outermost shell which make them stable.
Additional information:
Limitations of octet rule:
As hydrogen and helium both have only one and two electrons respectively in their outermost shell it is impossible for them to have eight electrons in their outermost shell. So there are many compounds which have less than eight electrons in their outermost shell but are still stable. Similarly, there are many compounds which have more than eight electrons in their outermost shell but are still stable.
Note: The octet rule is based on the concept that electronic configuration of the noble gases are most stable and they have eight electrons in their outermost orbital so every element should attain the similar electronic configuration to become stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




