
What is the octet rule of sulphur?
Answer
527.7k+ views
Hint: The octet rule is a bonding theory that is used to predict the structure of covalently bonded molecules. According to the rule, atoms tend to have eight electrons in their valence electron shells. The atom will share, gain, or lose electrons to have exactly eight electrons in valence shells.
Complete answer:
When we draw the Lewis dot structure of a molecule, we show the number of valence electrons by dots around the atoms and place them in a manner that atoms follow the octet rule.
But we often encounter the situation when the central atom expands octet or has more than 8 electrons around its valence shell. An element from Period 3 and below shows such exceptional behavior.
Notice that sulfur belongs to the third period of the periodic table and it has 3d subshell. Since, 3s, 3p, and 3d subshells are all in the same principal quantum shell, so they are all very close in terms of energy level.
Sulfur has a total of 16 electrons and out of these 16 electrons, 6 electrons are present in the valence shell. The electronic configuration of sulphur in the ground state is given below:
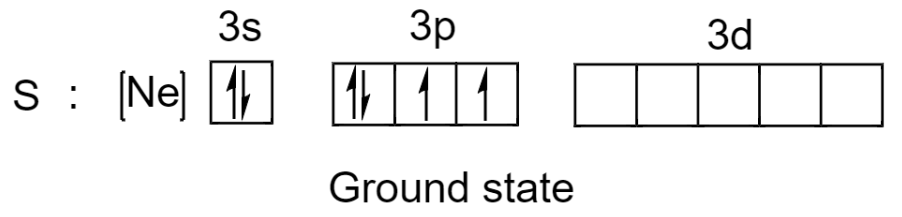
Now, sulphur can make use of its 2 unpaired electrons to form 2 covalent bonds and the remaining 4 electrons from its 2 lone pairs will give a total of 8 electrons.
But sulfur can unpair its electrons and promote one of its electrons to an empty 3d orbital. Since the energy difference between subshells in the same principal quantum shell is small, it requires very little energy for excitation to take place.
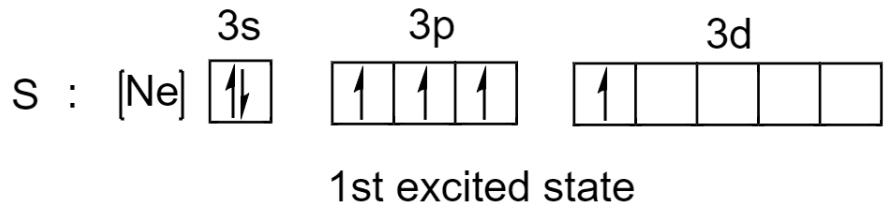
After excitation, we can see that there are now 4 unpaired electrons that can be used by sulphur to form 4 covalent bonds and 1 lone pair will give a total of 10 electrons in its valence shell.
Sulfur now has more than 8 electrons, we say that it has an "expanded octet".
Sulfur has one more electron pair in its 3s subshell so it can undergo excitation one more time. Now sulfur has expanded the octet to 12.
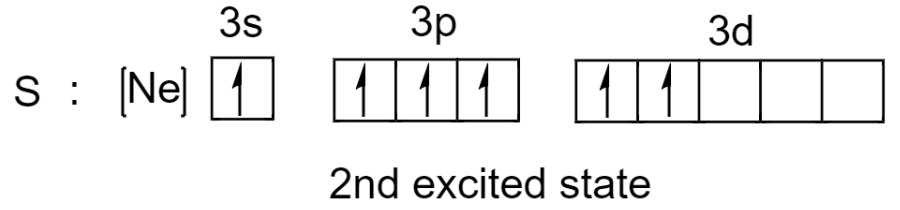
So in addition to being octet, sulfur has expanded the octet rule where it can possess 10 or 12 electrons in a valence shell.
Note:
Oxygen and other Period 2 elements do not use orbitals in the third principal quantum shell for bonding and cannot expand octet as it requires too much energy to unpair its electron and promote it to the 3s subshell from the 2p subshell.
Complete answer:
When we draw the Lewis dot structure of a molecule, we show the number of valence electrons by dots around the atoms and place them in a manner that atoms follow the octet rule.
But we often encounter the situation when the central atom expands octet or has more than 8 electrons around its valence shell. An element from Period 3 and below shows such exceptional behavior.
Notice that sulfur belongs to the third period of the periodic table and it has 3d subshell. Since, 3s, 3p, and 3d subshells are all in the same principal quantum shell, so they are all very close in terms of energy level.
Sulfur has a total of 16 electrons and out of these 16 electrons, 6 electrons are present in the valence shell. The electronic configuration of sulphur in the ground state is given below:

Now, sulphur can make use of its 2 unpaired electrons to form 2 covalent bonds and the remaining 4 electrons from its 2 lone pairs will give a total of 8 electrons.
But sulfur can unpair its electrons and promote one of its electrons to an empty 3d orbital. Since the energy difference between subshells in the same principal quantum shell is small, it requires very little energy for excitation to take place.

After excitation, we can see that there are now 4 unpaired electrons that can be used by sulphur to form 4 covalent bonds and 1 lone pair will give a total of 10 electrons in its valence shell.
Sulfur now has more than 8 electrons, we say that it has an "expanded octet".
Sulfur has one more electron pair in its 3s subshell so it can undergo excitation one more time. Now sulfur has expanded the octet to 12.

So in addition to being octet, sulfur has expanded the octet rule where it can possess 10 or 12 electrons in a valence shell.
Note:
Oxygen and other Period 2 elements do not use orbitals in the third principal quantum shell for bonding and cannot expand octet as it requires too much energy to unpair its electron and promote it to the 3s subshell from the 2p subshell.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




