
What is the structure of $ I{F_7} $ ?
Answer
491.4k+ views
Hint :Iodine hypo fluoride or iodine (VII) chloride are other names for $ I{F_7} $ . It's a strong-bonding interhalogen molecule. According to VSEPR theory, it has a pentagonal bi-pyramidal structure with $ s{p^3}{d^3} $ hybridization.
Complete Step By Step Answer:
The core atom I(iodine) is connected to 7 fluorine atoms via 7 sigma bonds in $ I{F_7} $ . So, in this case, the steric number is 7. As a result, I(iodine) hybridization in $ I{F_7} $ is $ s{p^3}{d^3} $ . As a result, both the electron pair and molecular geometry are pentagonal bi-pyramidal.
Let’s look how the structure comes:
Iodine(atomic no.=53)
$ \left[ {E.C} \right] = 4{d^{10}}5{s^2}5{p^5} $ where E.C=electronic configuration
Look at the following image:
In first excited state its electronic configuration changes:
$ \left[ {E.C} \right] = 5{s^1}5{p^3}5{d^3} $
Hence we can see that there are 7 fluorine molecules. One enters $ 5s $ , 3 can enter $ 5p $ and 3 will enter $ 5d $ .
Hence we can make out that the hybridization is $ s{p^3}{d^3} $ . And hence geometry is pentagonal bi-pyramidal
Let's look at the structure:
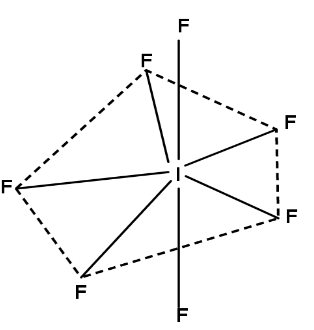
This is pentagonal bi-pyramidal. Also not every bonding angle is the same. Five electron pairs are on the same plane at a 72-degree angle, while the other two are perpendicular to the plane and make a 90-degree angle with it. Even bond lengths differ. The axial bonds are 186pm long, whereas the equatorial bonds are 179pm long.
Note :
There can be many ionization states of an element. But whenever we draw structures we should only consider the first excited state and calculate the hybridization.
Also The primary distinction between axial and equatorial positions is that axial bonds are vertical, whereas equatorial bonds are horizontal. The phrases axial and equatorial are critical when illustrating the real 3D orientation of chemical bonds.
It is not necessary to mention bond angles and bond lengths while drawing the structure.
Complete Step By Step Answer:
The core atom I(iodine) is connected to 7 fluorine atoms via 7 sigma bonds in $ I{F_7} $ . So, in this case, the steric number is 7. As a result, I(iodine) hybridization in $ I{F_7} $ is $ s{p^3}{d^3} $ . As a result, both the electron pair and molecular geometry are pentagonal bi-pyramidal.
Let’s look how the structure comes:
Iodine(atomic no.=53)
$ \left[ {E.C} \right] = 4{d^{10}}5{s^2}5{p^5} $ where E.C=electronic configuration
Look at the following image:
In first excited state its electronic configuration changes:
$ \left[ {E.C} \right] = 5{s^1}5{p^3}5{d^3} $
Hence we can see that there are 7 fluorine molecules. One enters $ 5s $ , 3 can enter $ 5p $ and 3 will enter $ 5d $ .
Hence we can make out that the hybridization is $ s{p^3}{d^3} $ . And hence geometry is pentagonal bi-pyramidal
Let's look at the structure:

This is pentagonal bi-pyramidal. Also not every bonding angle is the same. Five electron pairs are on the same plane at a 72-degree angle, while the other two are perpendicular to the plane and make a 90-degree angle with it. Even bond lengths differ. The axial bonds are 186pm long, whereas the equatorial bonds are 179pm long.
Note :
There can be many ionization states of an element. But whenever we draw structures we should only consider the first excited state and calculate the hybridization.
Also The primary distinction between axial and equatorial positions is that axial bonds are vertical, whereas equatorial bonds are horizontal. The phrases axial and equatorial are critical when illustrating the real 3D orientation of chemical bonds.
It is not necessary to mention bond angles and bond lengths while drawing the structure.
Recently Updated Pages
Why are manures considered better than fertilizers class 11 biology CBSE

Find the coordinates of the midpoint of the line segment class 11 maths CBSE

Distinguish between static friction limiting friction class 11 physics CBSE

The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE

The first National Commission on Labour NCL submitted class 11 social science CBSE

Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE

Trending doubts
10 examples of friction in our daily life

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Difference Between Prokaryotic Cells and Eukaryotic Cells

1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




