
What is the triple point?

Answer
577.5k+ views
Hint: The temperature and pressure at which all three phases coexist is known as triple point. A triple point is unique for every substance.
Complete step by step answer:
The given diagrams is represented as follows:
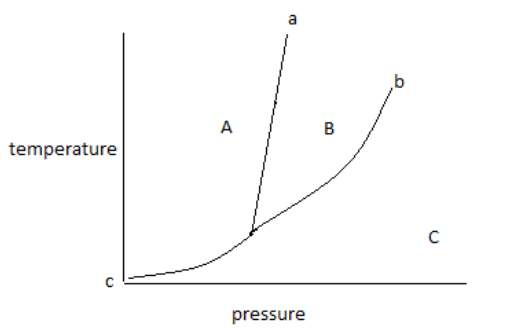
The above diagram is known as a phase diagram.
The phase diagram shows the temperature and pressure conditions for the conversion of different phases into each other and the triple point at which all the phases coexist.
The phase diagram is plotted between temperature and pressure.
The given phase diagram is for one component system.
The phase diagram contains areas, curves, and triple points.
In the above phase diagram, area A is for the solid phase, area B is for the liquid phase and area C is for the gas phase. Area shows the existence of one phase only in a temperature and pressure value. On changing, temperature and pressure in the area phase does not change.
Curve ‘a’ represents the fusion curve, curve ‘b’ represents the vaporization curve and curve ‘c’ represents the sublimation curve. The curve shows the existence of two phases at equilibrium at the temperature and pressure values. Any temperature and pressure values at the curve, two phases coexist.
1. The fusion curve shows the coexistence of solid and liquid.
2. The vaporization curve shows the coexistence of liquid and gas.
3. The sublimation curve shows the coexistence of solid and gas.
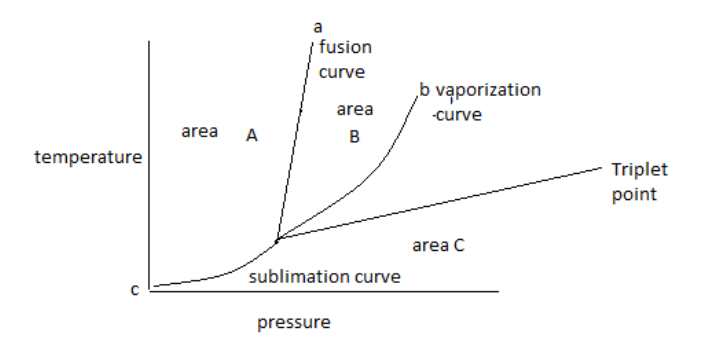
A triple point is the point at which three curves a, b and c met with each other. Therefore, the meeting point of all curves in the diagram shows the triple point.
Note:
A triple point can be identified in the diagram as the point at which three curves join each other. At a triple point all three phases’ remains in equilibrium. Some substances do not coexist in all three phases at any temperature and pressure value, that substance does not have a triple point.
Complete step by step answer:
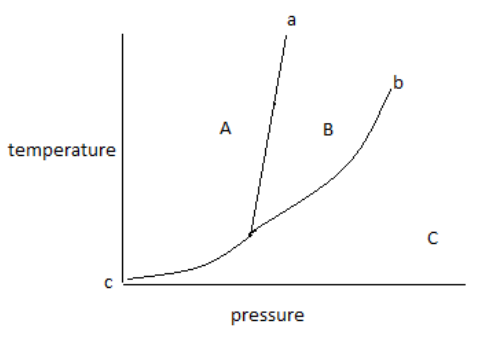
The given diagrams is represented as follows:

The above diagram is known as a phase diagram.
The phase diagram shows the temperature and pressure conditions for the conversion of different phases into each other and the triple point at which all the phases coexist.
The phase diagram is plotted between temperature and pressure.
The given phase diagram is for one component system.
The phase diagram contains areas, curves, and triple points.
In the above phase diagram, area A is for the solid phase, area B is for the liquid phase and area C is for the gas phase. Area shows the existence of one phase only in a temperature and pressure value. On changing, temperature and pressure in the area phase does not change.
Curve ‘a’ represents the fusion curve, curve ‘b’ represents the vaporization curve and curve ‘c’ represents the sublimation curve. The curve shows the existence of two phases at equilibrium at the temperature and pressure values. Any temperature and pressure values at the curve, two phases coexist.
1. The fusion curve shows the coexistence of solid and liquid.
2. The vaporization curve shows the coexistence of liquid and gas.
3. The sublimation curve shows the coexistence of solid and gas.

A triple point is the point at which three curves a, b and c met with each other. Therefore, the meeting point of all curves in the diagram shows the triple point.
Note:
A triple point can be identified in the diagram as the point at which three curves join each other. At a triple point all three phases’ remains in equilibrium. Some substances do not coexist in all three phases at any temperature and pressure value, that substance does not have a triple point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




