
What's the role of \[FeC{l_3}\] in Halogenation of Benzene?
Answer
478.5k+ views
Hint: Halogenation is a reaction that happens when one or more halogens are added to a substance. Halogens comprise the seventh column within the table and include fluorine, chlorine, bromine, iodine, and astatine. The resulting product of a halogenation reaction is understood as a halogenated compound.
Complete Answer:
\[FeC{l_3}\] , Iron Chloride, maybe a good Lewis acid. Therefore, when benzene reacts with halogens within the presence of \[FeC{l_3}\] , aryl halides are formed. This is often the method of halogenation of benzene.
Halogens aren't electrophilic enough to interrupt the aromaticity of benzenes as benzenes are normally unreactive. Hence, Halogen needs the assistance and aid of Lewis Acidic Catalysts to activate it to become a really strong electrophile.
\[Iron\left( {III} \right)chloride\] \[\left( {FeC{l_3}} \right)\] may be a good Lewis acid and has been very widely and effectively employed as a catalyst during a variety of organic chemical reactions. \[P{h_2}S{e_2}\] occurs via an EAS mechanism which is amid auto-redox processes on the selenium center facilitated by \[FeC{l_3}\] .
Benzene reacts with chlorine or bromine in an electrophilic substitution reaction, but only within the presence of a catalyst. The catalyst is either aluminum chloride (or aluminum bromide if you're reacting benzene with bromine) or iron.
Note:
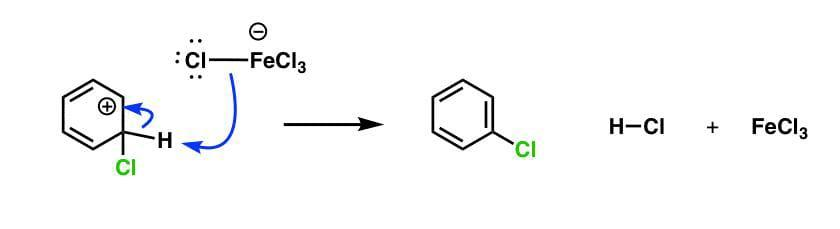
When benzene is treated with chlorine in presence of a Lewis acid like \[FeC{l_3}\] , then instead of chemical reaction substitution reaction takes place, during which one chlorine atom is replaced by one atom from the benzene formula during this substitution reaction chlorine is polarized by the Lewis base (i.e. \[FeC{l_3}\] here), which introduces a partial charge on the chlorine atom.
Complete Answer:
\[FeC{l_3}\] , Iron Chloride, maybe a good Lewis acid. Therefore, when benzene reacts with halogens within the presence of \[FeC{l_3}\] , aryl halides are formed. This is often the method of halogenation of benzene.
Halogens aren't electrophilic enough to interrupt the aromaticity of benzenes as benzenes are normally unreactive. Hence, Halogen needs the assistance and aid of Lewis Acidic Catalysts to activate it to become a really strong electrophile.
\[Iron\left( {III} \right)chloride\] \[\left( {FeC{l_3}} \right)\] may be a good Lewis acid and has been very widely and effectively employed as a catalyst during a variety of organic chemical reactions. \[P{h_2}S{e_2}\] occurs via an EAS mechanism which is amid auto-redox processes on the selenium center facilitated by \[FeC{l_3}\] .
Benzene reacts with chlorine or bromine in an electrophilic substitution reaction, but only within the presence of a catalyst. The catalyst is either aluminum chloride (or aluminum bromide if you're reacting benzene with bromine) or iron.

Note:
When benzene is treated with chlorine in presence of a Lewis acid like \[FeC{l_3}\] , then instead of chemical reaction substitution reaction takes place, during which one chlorine atom is replaced by one atom from the benzene formula during this substitution reaction chlorine is polarized by the Lewis base (i.e. \[FeC{l_3}\] here), which introduces a partial charge on the chlorine atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




