
Which among the following is a non-benzenoid aromatic compound?
A. o-xylene
B. Phenanthrene
C. Indole
D. Thiophene
Answer
592.8k+ views
Hint:Aromaticity means a conjugated system made of alternating single and double bonds in a ring. This conjugation allows the electrons in the molecule to be delocalized around the ring thus, increasing the molecule's stability.
Complete step by step answer:
To find the answer to this question, we should know what non-benzenoid compounds are, these are aromatic compounds which have highly unsaturated rings other than benzene rings. Aromatic compounds are cyclic and planar structures with a ring of resonance bonds. Aromatic molecules are very stable. Let us discuss the options one by one to find non-benzenoid aromatic compound:
A. o-xylene: It is also known as ortho-xylene is an aromatic compound with the formula ${{\text{C}}_{6}}{{\text{H}}_{4}}{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}$. The two methyl groups bonded to adjacent carbon atoms of a benzene ring. It is an isomer of m-xylene and p-xylene and their mixture is called xylene or xylenes. Its structure is
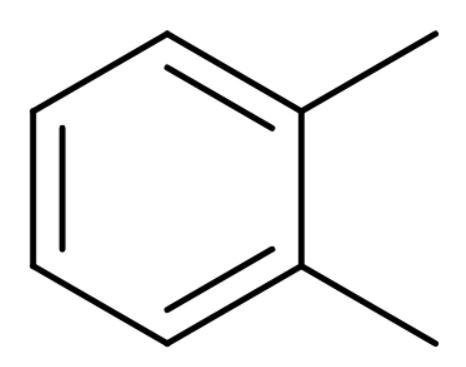
By structure it is clear that it is a benzenoid compound.
B. Phenanthrene: A polycyclic aromatic compound composed of three fused benzene rings. It is made up of phenyl and anthracene. Its structure is

As it is a fusion of benzene rings then, it is a benzenoid compound.
C. Indole: Its molecular formula is ${{\text{C}}_{8}}{{\text{H}}_{7}}\text{N}$ and it is an aromatic heterocyclic organic compound. Its structure is bicyclic consisting of a six-membered benzene ring attached to a five-membered pyrrole ring. Its structure is

The lone pair on nitrogen is in resonance with the rings and satisfies Huckel’s Rule (the compound should have $\left( 4\text{n}+\text{2} \right)\pi \text{ }{{\text{e}}^{-}}$ in it). This compound has 10 $\pi {{\text{e}}^{-}}$in it which satisfies the rule. It has benzene attached to it. So, it is a benzenoid aromatic compound.
D. Thiophene: Thiophene is aromatic, heterocyclic compound with molecular formula ${{\text{C}}_{4}}{{\text{H}}_{4}}\text{S}$. Consisting of a planar five-membered ring. It resembles benzene. The lone pair on sulphur is delocalized in the pi electron system. Its structure is

It has 6 $\pi {{\text{e}}^{-}}$ inside the ring. It is aromatic but it is not attached to any benzene ring. So, it is a non-benzenoid compound.
The correct answer to this question is option ‘d’ (Thiophene).
Note:
By using Huckel’s Rule, we can check whether a compound is aromatic or not. The conditions of aromaticity are (i) should have $\left( 4\text{n}+\text{2} \right)\pi \text{ }{{\text{e}}^{-}}$in it. (ii) should have cyclic and planar structure and (iii) the electrons present should be inside the ring only.
Complete step by step answer:
To find the answer to this question, we should know what non-benzenoid compounds are, these are aromatic compounds which have highly unsaturated rings other than benzene rings. Aromatic compounds are cyclic and planar structures with a ring of resonance bonds. Aromatic molecules are very stable. Let us discuss the options one by one to find non-benzenoid aromatic compound:
A. o-xylene: It is also known as ortho-xylene is an aromatic compound with the formula ${{\text{C}}_{6}}{{\text{H}}_{4}}{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}$. The two methyl groups bonded to adjacent carbon atoms of a benzene ring. It is an isomer of m-xylene and p-xylene and their mixture is called xylene or xylenes. Its structure is

By structure it is clear that it is a benzenoid compound.
B. Phenanthrene: A polycyclic aromatic compound composed of three fused benzene rings. It is made up of phenyl and anthracene. Its structure is

As it is a fusion of benzene rings then, it is a benzenoid compound.
C. Indole: Its molecular formula is ${{\text{C}}_{8}}{{\text{H}}_{7}}\text{N}$ and it is an aromatic heterocyclic organic compound. Its structure is bicyclic consisting of a six-membered benzene ring attached to a five-membered pyrrole ring. Its structure is

The lone pair on nitrogen is in resonance with the rings and satisfies Huckel’s Rule (the compound should have $\left( 4\text{n}+\text{2} \right)\pi \text{ }{{\text{e}}^{-}}$ in it). This compound has 10 $\pi {{\text{e}}^{-}}$in it which satisfies the rule. It has benzene attached to it. So, it is a benzenoid aromatic compound.
D. Thiophene: Thiophene is aromatic, heterocyclic compound with molecular formula ${{\text{C}}_{4}}{{\text{H}}_{4}}\text{S}$. Consisting of a planar five-membered ring. It resembles benzene. The lone pair on sulphur is delocalized in the pi electron system. Its structure is

It has 6 $\pi {{\text{e}}^{-}}$ inside the ring. It is aromatic but it is not attached to any benzene ring. So, it is a non-benzenoid compound.
The correct answer to this question is option ‘d’ (Thiophene).
Note:
By using Huckel’s Rule, we can check whether a compound is aromatic or not. The conditions of aromaticity are (i) should have $\left( 4\text{n}+\text{2} \right)\pi \text{ }{{\text{e}}^{-}}$in it. (ii) should have cyclic and planar structure and (iii) the electrons present should be inside the ring only.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




