
Which atomic orbitals of which subshells have a dumbbell shape?
Answer
471.3k+ views
Hint: We need to study atomic orbitals and their shapes in regards to subshells. For a better understanding of the geometry of electron orbitals, quantum theory and other characteristics of electron orbitals are used.
Complete answer:
One of three quantum numbers used to characterise an orbital, the main quantum number is one of the three quantum numbers. Unlike a regular orbit, atomic orbitals are broad regions in an atom where electrons are most likely to reside. When an electron is found in three-dimensional space surrounding a nucleus, a quantum mechanical model determines its probability. The angular momentum quantum number, $l$, is another quantum number. It is an integer that takes the values\[l{\text{ }} = {\text{ }}0,{\text{ }}1,{\text{ }}2\],..., \[n{\text{ }}-{\text{ }}1\]and specifies the form of the orbital. This means that an orbital with \[n{\text{ }} = {\text{ }}1\]may only have one $l$ value, \[l{\text{ }} = {\text{ }}0\], but an orbital with \[n{\text{ }} = {\text{ }}2\]can have \[l{\text{ }} = {\text{ }}0\]and\[l{\text{ }} = {\text{ }}1\], and so on. The orbital's overall size and energy are defined by the main quantum number. The orbital's form is determined by the$l$ value. A subshell is formed by orbitals with the same $l$value. Furthermore, the angular momentum of an electron in this orbital is proportional to the angular momentum quantum number.
The $s$ subshells have a sphere-like form. The $s$orbital is present in both the $1n$ and $2n$ main shells, although the sphere in the $2n$ orbital is bigger. Each of the spheres represents a single orbital. Three dumbbell-shaped orbitals make up $p$ subshells. Shell $1$ does not have a $p$ subshell, but principal shell $2n$ does.
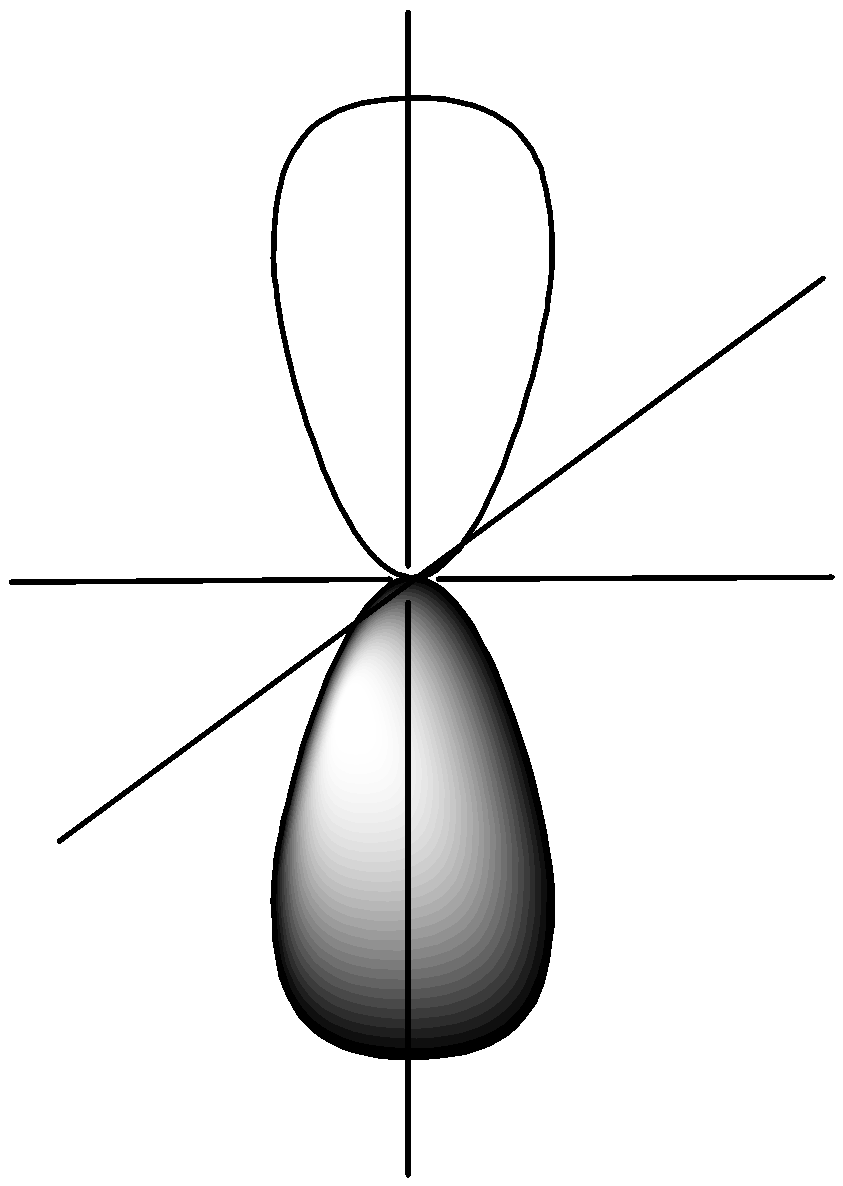
Fig: dumbbell-shaped orbitals make up $p$ subshells (x,y and z axis)
Note:
Note that $s$ orbitals are orbitals with\[l{\text{ }} = {\text{ }}0\].The $p$ orbitals are represented by the value \[l{\text{ }} = {\text{ }}1.\]$p$ orbitals form a $p$ subshell for a given $n$ (e.g., \[3p\]for\[n{\text{ }} = {\text{ }}3\]). The \[d\]orbitals are those with\[l{\text{ }} = {\text{ }}2\], followed by the \[f - ,{\text{ }}g - ,\]and \[h - \]orbitals with \[l{\text{ }} = {\text{ }}3,{\text{ }}4,{\text{ }}5,\]and higher values.
Complete answer:
One of three quantum numbers used to characterise an orbital, the main quantum number is one of the three quantum numbers. Unlike a regular orbit, atomic orbitals are broad regions in an atom where electrons are most likely to reside. When an electron is found in three-dimensional space surrounding a nucleus, a quantum mechanical model determines its probability. The angular momentum quantum number, $l$, is another quantum number. It is an integer that takes the values\[l{\text{ }} = {\text{ }}0,{\text{ }}1,{\text{ }}2\],..., \[n{\text{ }}-{\text{ }}1\]and specifies the form of the orbital. This means that an orbital with \[n{\text{ }} = {\text{ }}1\]may only have one $l$ value, \[l{\text{ }} = {\text{ }}0\], but an orbital with \[n{\text{ }} = {\text{ }}2\]can have \[l{\text{ }} = {\text{ }}0\]and\[l{\text{ }} = {\text{ }}1\], and so on. The orbital's overall size and energy are defined by the main quantum number. The orbital's form is determined by the$l$ value. A subshell is formed by orbitals with the same $l$value. Furthermore, the angular momentum of an electron in this orbital is proportional to the angular momentum quantum number.
The $s$ subshells have a sphere-like form. The $s$orbital is present in both the $1n$ and $2n$ main shells, although the sphere in the $2n$ orbital is bigger. Each of the spheres represents a single orbital. Three dumbbell-shaped orbitals make up $p$ subshells. Shell $1$ does not have a $p$ subshell, but principal shell $2n$ does.

Fig: dumbbell-shaped orbitals make up $p$ subshells (x,y and z axis)
Note:
Note that $s$ orbitals are orbitals with\[l{\text{ }} = {\text{ }}0\].The $p$ orbitals are represented by the value \[l{\text{ }} = {\text{ }}1.\]$p$ orbitals form a $p$ subshell for a given $n$ (e.g., \[3p\]for\[n{\text{ }} = {\text{ }}3\]). The \[d\]orbitals are those with\[l{\text{ }} = {\text{ }}2\], followed by the \[f - ,{\text{ }}g - ,\]and \[h - \]orbitals with \[l{\text{ }} = {\text{ }}3,{\text{ }}4,{\text{ }}5,\]and higher values.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




