
Which chloro derivative of benzene undergoes hydrolysis most readily with aq. NaOH?
(A)-
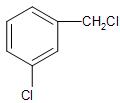
(B)-
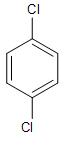
(C)-
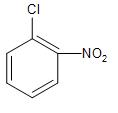
(D)-
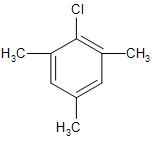




Answer
578.1k+ views
Hint: The reaction with the aqueous NaOH, with the strong nucleophile of hydroxide ion will produce a nucleophilic substitution reaction on the benzene ring. The stability of the carbocation formed will determine the ease of attack of the nucleophile.
Complete step by step answer:
In the given chloro derivatives of the benzene, on the hydrolysis with aqueous sodium hydroxide, it undergoes the nucleophilic substitution reaction by the hydroxide ion acting as the nucleophile. The ease with which the reaction will occur, will be determined by the stability of the carbocation formed as the leaving group $(C{{l}^{-}})$ departs from the compound.
In case of compound (A), The chlorine atom attached to the benzylic carbon, will undergo the nucleophilic substitution through ${{S}_{N}}2$ mechanism, leading to the formation of a carbocation intermediate. Due to the presence of the positive charge over the benzylic carbon, which is stabilised by the resonance in the benzene ring will be the most favoured.
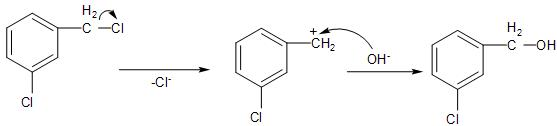
Whereas, in the remaining three compounds, the leaving group will form a phenyl carbocation on the benzene ring, that is the phenolic carbon. This positive charge on the phenolic carbon, is present on the sp- hybridised carbon atom, which is not stabilised by the aromatic ring.
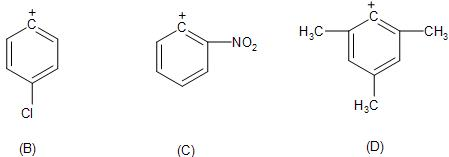
But the presence of the electron-donating groups like the methyl group at the ortho- and para-position with respect to the leaving group, will increase the electron-density over the ring and stabilise it to some extent, whereas the electron-withdrawing group like the $(-N{{O}_{2}})$ will further decrease the stability of the carbocation.
Therefore, the chloro derivative of benzene undergoing hydrolysis most readily with aq. NaOH will be option (A)-
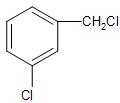
So, the correct answer is “Option A”.
Note: Due to the greater s-character in the sp- hybridised orbital in the phenyl carbocation, with a positive charge it faces greater repulsion from the nucleus and is highly unstable.
Also, the remaining three compounds show the aromatic nucleophilic substitution reaction, with the addition-elimination reaction mechanism.
Complete step by step answer:
In the given chloro derivatives of the benzene, on the hydrolysis with aqueous sodium hydroxide, it undergoes the nucleophilic substitution reaction by the hydroxide ion acting as the nucleophile. The ease with which the reaction will occur, will be determined by the stability of the carbocation formed as the leaving group $(C{{l}^{-}})$ departs from the compound.
In case of compound (A), The chlorine atom attached to the benzylic carbon, will undergo the nucleophilic substitution through ${{S}_{N}}2$ mechanism, leading to the formation of a carbocation intermediate. Due to the presence of the positive charge over the benzylic carbon, which is stabilised by the resonance in the benzene ring will be the most favoured.

Whereas, in the remaining three compounds, the leaving group will form a phenyl carbocation on the benzene ring, that is the phenolic carbon. This positive charge on the phenolic carbon, is present on the sp- hybridised carbon atom, which is not stabilised by the aromatic ring.

But the presence of the electron-donating groups like the methyl group at the ortho- and para-position with respect to the leaving group, will increase the electron-density over the ring and stabilise it to some extent, whereas the electron-withdrawing group like the $(-N{{O}_{2}})$ will further decrease the stability of the carbocation.
Therefore, the chloro derivative of benzene undergoing hydrolysis most readily with aq. NaOH will be option (A)-

So, the correct answer is “Option A”.
Note: Due to the greater s-character in the sp- hybridised orbital in the phenyl carbocation, with a positive charge it faces greater repulsion from the nucleus and is highly unstable.
Also, the remaining three compounds show the aromatic nucleophilic substitution reaction, with the addition-elimination reaction mechanism.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




