
Which choice is a correct Lewis Structure for $\text{BeC}{{\text{l}}_{2}}$?
A)
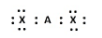
B)
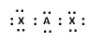
C)

D)

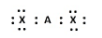
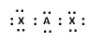


Answer
548.1k+ views
Hint:
The Lewis electron dot structure is a representation of the valence electrons of the atoms done by using dots around the central atom. A shared electron pair represents a covalent bond.
Complete step by step solution:
The electronic configuration of beryllium in $\text{BeC}{{\text{l}}_{2}}$ is, $\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}$ so it has two electrons in its valence shell and that for chlorine is $\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{5}}}$ so chlorine has seven electrons its outermost shell.
A) In this structure, the central element A has four electrons, two from its own valance shell and the other two form the valence shells of chlorine. Also the octet of the chlorine atoms are satisfied after having 8 electrons.
B) In this structure, the central element A has eight electrons, two from its own valence shell two form the valence shells of chlorine, but the rest of the four electrons cannot be accounted for. The octet of the chlorine atoms are satisfied after having 8 electrons. So this structure of $\text{BeC}{{\text{l}}_{2}}$is not possible at all.
C) In this structure there are three atoms of chlorine which is not possible looking at the molecular formula of $\text{BeC}{{\text{l}}_{2}}$.
D) Again, in this structure also there are three atoms of chlorine which is not possible from the molecular formula of the salt.
Hence, it can be concluded from here that the correct electron dot structure of $\text{BeC}{{\text{l}}_{2}}$ is the structure A.
Note:
The naming is done after Gilbert N. Lewis who introduced it in 1916, in the article, “ The atoms and the Molecules”. The electron dot structures extend the concept of electron dot diagram by adding lines between the atoms to represent shared pairs in a chemical bond.
The Lewis electron dot structure is a representation of the valence electrons of the atoms done by using dots around the central atom. A shared electron pair represents a covalent bond.
Complete step by step solution:
The electronic configuration of beryllium in $\text{BeC}{{\text{l}}_{2}}$ is, $\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}$ so it has two electrons in its valence shell and that for chlorine is $\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{5}}}$ so chlorine has seven electrons its outermost shell.
A) In this structure, the central element A has four electrons, two from its own valance shell and the other two form the valence shells of chlorine. Also the octet of the chlorine atoms are satisfied after having 8 electrons.
B) In this structure, the central element A has eight electrons, two from its own valence shell two form the valence shells of chlorine, but the rest of the four electrons cannot be accounted for. The octet of the chlorine atoms are satisfied after having 8 electrons. So this structure of $\text{BeC}{{\text{l}}_{2}}$is not possible at all.
C) In this structure there are three atoms of chlorine which is not possible looking at the molecular formula of $\text{BeC}{{\text{l}}_{2}}$.
D) Again, in this structure also there are three atoms of chlorine which is not possible from the molecular formula of the salt.
Hence, it can be concluded from here that the correct electron dot structure of $\text{BeC}{{\text{l}}_{2}}$ is the structure A.
Note:
The naming is done after Gilbert N. Lewis who introduced it in 1916, in the article, “ The atoms and the Molecules”. The electron dot structures extend the concept of electron dot diagram by adding lines between the atoms to represent shared pairs in a chemical bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




