
Which combination is best explained by the coordinate covalent bond?
(A)- ${{H}_{2}}+{{I}_{2}}$
(B)- $Mg+\frac{1}{2}{{O}_{2}}$
(C)- Cl + Cl
(D)- ${{H}^{+}}+{{H}_{2}}O$
Answer
591.9k+ views
Hint: A bond formed by sharing a pair of electrons by two atoms from one atom only is called a coordinate bond or a dative covalent bond. Hydronium ions have two O-H covalent bonds and one O-H coordinate bond which after the formation of $O\to {{H}^{+}}$ co-ordinate bond becomes identical to the two O-H covalent bonds. Hence in ${{H}_{3}}{{O}^{+}}$ ion all the three bonds are identical.
Complete answer:
-A covalent bond is a kind of 2-center, 2-electron bond in which the two electrons derived are from the same atom.
-This type of bonding is seen in coordination compounds where a metal ion is bonded to ligands.
- In the first option, the combination given is ${{H}_{2}}+{{I}_{2}}$ which forms 2HI. The HI molecule exists with a covalent molecule with H and I sharing one electron each.
-In the second option, the combination given is $Mg+\frac{1}{2}{{O}_{2}}$ which forms MgO. In MgO molecules, oxygen gains two electrons to complete its octet and the bond formed is an ionic bond.
-In the third option, the combination given is Cl + Cl which forms a chlorine molecule. In $C{{l}_{2}}$ , both atoms share and tightly hold each other’s electrons and form a covalent bond.
-In the fourth option, the combination given is ${{H}^{+}}+{{H}_{2}}O$ which forms ${{H}_{3}}{{O}^{+}}$ . The bonding in the hydronium ion is a coolant bond, in which one electron comes from the oxygen and one comes from the hydrogen.
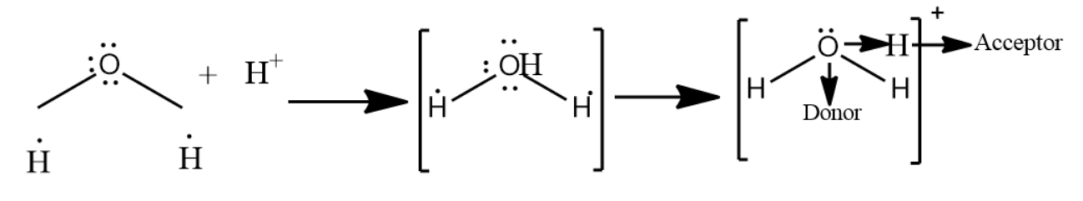
Therefore, the best combination explaining the coordinate bond is the combination of ${{H}^{+}}+{{H}_{2}}O$
So, the correct answer is “Option D”.
Note: Hydronium is the common name of aqueous cation which is a type of oxonium ion produced by protonation of water. Hydronium ion has a trigonal pyramidal molecular geometry with the oxygen atom at its apex. The H-O-H bond angle is approximate $113{}^\circ $ .
Complete answer:
-A covalent bond is a kind of 2-center, 2-electron bond in which the two electrons derived are from the same atom.
-This type of bonding is seen in coordination compounds where a metal ion is bonded to ligands.
- In the first option, the combination given is ${{H}_{2}}+{{I}_{2}}$ which forms 2HI. The HI molecule exists with a covalent molecule with H and I sharing one electron each.
-In the second option, the combination given is $Mg+\frac{1}{2}{{O}_{2}}$ which forms MgO. In MgO molecules, oxygen gains two electrons to complete its octet and the bond formed is an ionic bond.
-In the third option, the combination given is Cl + Cl which forms a chlorine molecule. In $C{{l}_{2}}$ , both atoms share and tightly hold each other’s electrons and form a covalent bond.
-In the fourth option, the combination given is ${{H}^{+}}+{{H}_{2}}O$ which forms ${{H}_{3}}{{O}^{+}}$ . The bonding in the hydronium ion is a coolant bond, in which one electron comes from the oxygen and one comes from the hydrogen.

Therefore, the best combination explaining the coordinate bond is the combination of ${{H}^{+}}+{{H}_{2}}O$
So, the correct answer is “Option D”.
Note: Hydronium is the common name of aqueous cation which is a type of oxonium ion produced by protonation of water. Hydronium ion has a trigonal pyramidal molecular geometry with the oxygen atom at its apex. The H-O-H bond angle is approximate $113{}^\circ $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




