
Which gas will come out when a mixture of methane, ethylene, and acetylene is passed through a Wolf bottle containing ammoniacal cuprous chloride?
A. methane
B. acetylene
C. a mixture of methane and ethylene
D. organic mixture
Answer
569.4k+ views
Hint: Ammoniacal cuprous chloride has a molecular formula $2\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]Cl$ . It is an organic compound that reacts with terminal alkynes. This solution is used to test the presence of a triple bond in an organic compound. Ammoniacal cuprous chloride reacts with terminal alkynes to form a red precipitate of copper salt.
Complete Step by step answer: Ammoniacal cuprous chloride is a solution of cuprous chloride in ammonia. It is prepared by mixing cuprous chloride in water and ammonia till we obtain a blue-colored solution. This solution is used to test the presence of a triple bond in an organic compound. Ammoniacal cuprous chloride reacts with terminal alkynes to form a red precipitate of copper salt.
Now we are given a mixture of methane, ethylene, and acetylene. Let us see what happens when this mixture is passed through ammoniacal cuprous chloride solution
Methane is an organic compound with a molecular formula $C{H_4}$. It is a form of gas and is the simplest hydrocarbon. When it is passed through ammoniacal cuprous chloride, no reaction will occur. Because there is no triple bond present
$C{H_4} + 2\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]Cl \to $No reaction
Similarly, ethylene has a molecular formula ${C_2}{H_4}$ . It has a structure $_2HC = C{H_2}$ . When it is passed through ammoniacal cuprous chloride, no reaction will occur. Because there is no triple bond present
${C_2}{H_2} + 2\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]Cl \to $No reaction
Acetylene has a molecular formula ${C_2}{H_2}$ and structure . Since it has a triple bond it will undergo a reaction with ammoniacal cuprous chloride.
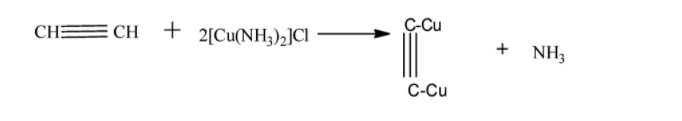
Thus the correct option is $C$ because methane and ethylene are forms of gases and no reaction is occurring with ammoniacal cuprous chloride.
Note: It is important to know what are terminal alkynes. Terminal alkynes are those alkynes in which at least one $H$ atom is bonded to a triple bond carbon atom. Similarly, we have terminal alkenes in which a double bond is at the end of the carbon chain.
Complete Step by step answer: Ammoniacal cuprous chloride is a solution of cuprous chloride in ammonia. It is prepared by mixing cuprous chloride in water and ammonia till we obtain a blue-colored solution. This solution is used to test the presence of a triple bond in an organic compound. Ammoniacal cuprous chloride reacts with terminal alkynes to form a red precipitate of copper salt.
Now we are given a mixture of methane, ethylene, and acetylene. Let us see what happens when this mixture is passed through ammoniacal cuprous chloride solution
Methane is an organic compound with a molecular formula $C{H_4}$. It is a form of gas and is the simplest hydrocarbon. When it is passed through ammoniacal cuprous chloride, no reaction will occur. Because there is no triple bond present
$C{H_4} + 2\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]Cl \to $No reaction
Similarly, ethylene has a molecular formula ${C_2}{H_4}$ . It has a structure $_2HC = C{H_2}$ . When it is passed through ammoniacal cuprous chloride, no reaction will occur. Because there is no triple bond present
${C_2}{H_2} + 2\left[ {Cu{{\left( {N{H_3}} \right)}_2}} \right]Cl \to $No reaction
Acetylene has a molecular formula ${C_2}{H_2}$ and structure . Since it has a triple bond it will undergo a reaction with ammoniacal cuprous chloride.

Thus the correct option is $C$ because methane and ethylene are forms of gases and no reaction is occurring with ammoniacal cuprous chloride.
Note: It is important to know what are terminal alkynes. Terminal alkynes are those alkynes in which at least one $H$ atom is bonded to a triple bond carbon atom. Similarly, we have terminal alkenes in which a double bond is at the end of the carbon chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




