
Which group can be represented by the Lewis dot structure shown in the figure?
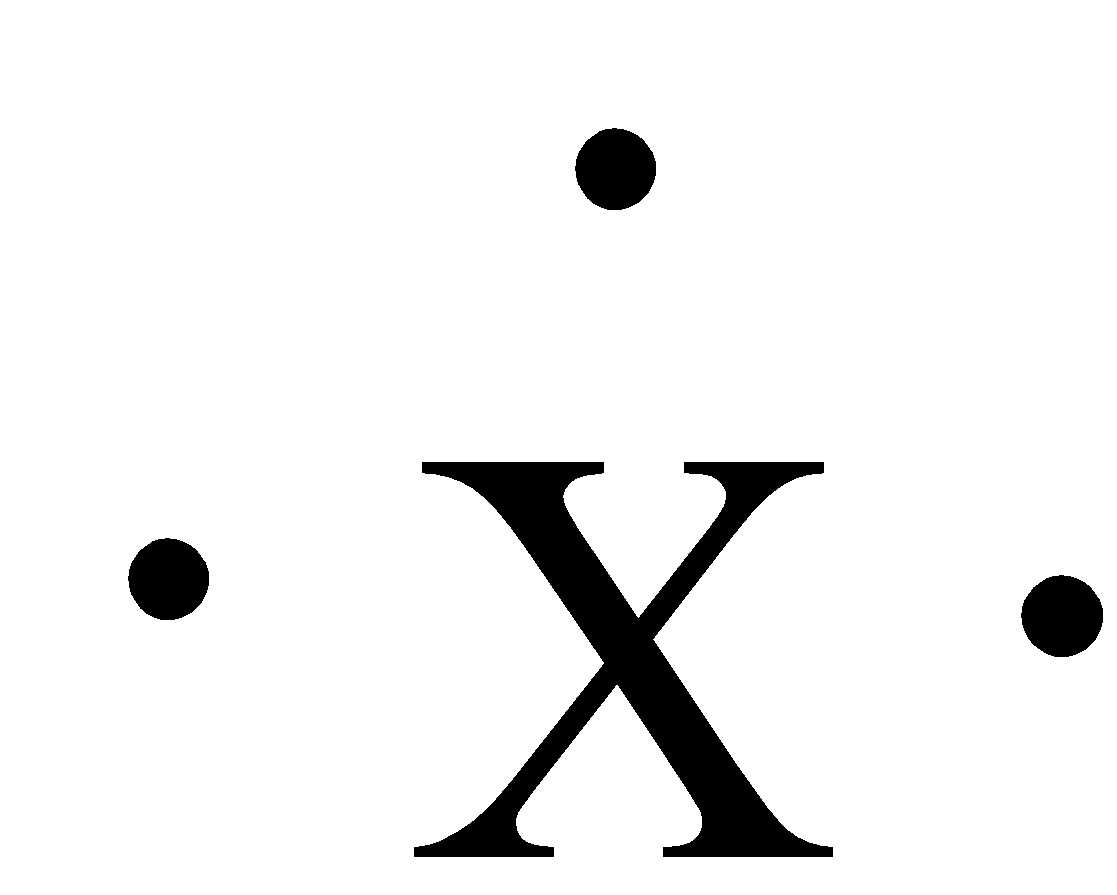
(a)- Group I A
(b)- Group II A
(c)- Group III A
(d)- Group VI A
(e)- Group VII A

Answer
512.4k+ views
Hint: The naming of the group in the given options was given by Mendeleev in his Mendeleev's periodic table. If the element has three dots around the element means the element has three valence electrons so, it will belong to group 13.
Complete answer:
Lewis dot structure is used to tell the number of valence electrons in the element. And it is represented by putting the dots equal to the number of valence electrons. For an element, write its symbol and put the number of dots around the symbol.
The naming of the group in the given options was given by Mendeleev in his Mendeleev's periodic table. The given options are a group I A is known as group 1, group II A is known as group 2, group III A is known as group 13, group VI A is known as group 16, and group VII A is known as group 17.
The given figure in the question is:
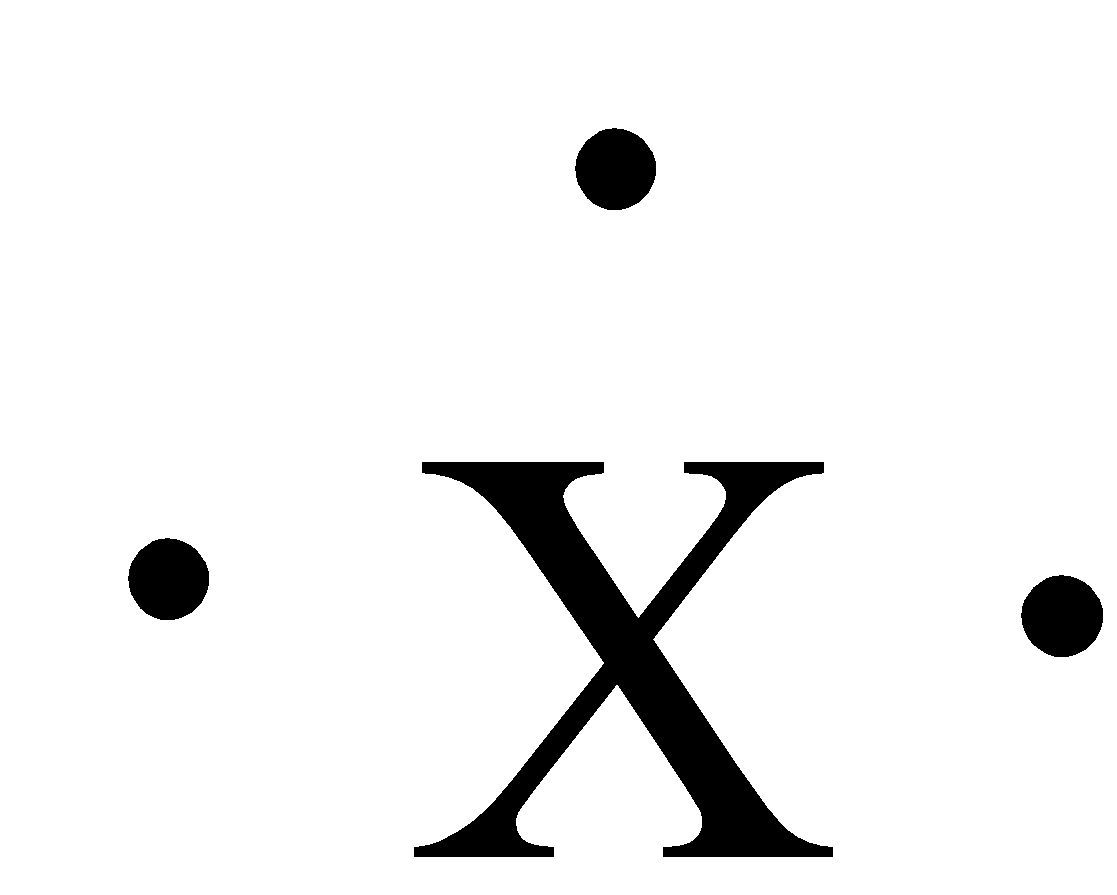
The numbers of dots around the symbol are three. Group 13 has 3 valence electrons, 2 from the s-orbital and 1 from the p-orbital. The elements that have this Lewis dot structure are boron, aluminium, gallium, indium, and thallium. So, this Lewis dot structure is for Group III A.
Therefore, the correct answer is an option (c)- Group III A.
Note:
Valence electrons are present in the valence shell and the valence shell is decided by the shell number so, all the subshells in the shell will be considered. For example, if the last shell is 2 and electrons are in 2s and 2p, then both of them will be considered for valence electrons.
Complete answer:
Lewis dot structure is used to tell the number of valence electrons in the element. And it is represented by putting the dots equal to the number of valence electrons. For an element, write its symbol and put the number of dots around the symbol.
The naming of the group in the given options was given by Mendeleev in his Mendeleev's periodic table. The given options are a group I A is known as group 1, group II A is known as group 2, group III A is known as group 13, group VI A is known as group 16, and group VII A is known as group 17.
The given figure in the question is:

The numbers of dots around the symbol are three. Group 13 has 3 valence electrons, 2 from the s-orbital and 1 from the p-orbital. The elements that have this Lewis dot structure are boron, aluminium, gallium, indium, and thallium. So, this Lewis dot structure is for Group III A.
Therefore, the correct answer is an option (c)- Group III A.
Note:
Valence electrons are present in the valence shell and the valence shell is decided by the shell number so, all the subshells in the shell will be considered. For example, if the last shell is 2 and electrons are in 2s and 2p, then both of them will be considered for valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




