
Which group of elements does not show a diagonal relationship?
A.$Li,Mg$
B.$Be,Al$
C.$B,Si$
D.$C,P$
Answer
517.2k+ views
Hint: We have to know that, in inclining relationships in S block components exist between adjoining components which are situated in the second and third times of the intermittent table. The properties of S block components shift essentially when contrasted with different components of the sub-bunch they have a place with.
Complete answer:
We have to know that the above points,
The corner to corner neighbors show a ton of similitudes. Such a relationship is displayed as you move left to right and down the gathering; the occasional table has contradicting factors.
A few connections happen due to the various manners by which numerous nuclear properties shift down gatherings and across times of the Periodic Table.
Notwithstanding the gathering and period connections, the components of s and p-block components likewise show corner to corner connections.
On moving corner to corner across the occasional table, the components show certain likenesses which are anyway undeniably less articulated than the similitudes inside a gathering. The corner to corner relationship is especially perceptible in the components of the second and third times of the intermittent table.
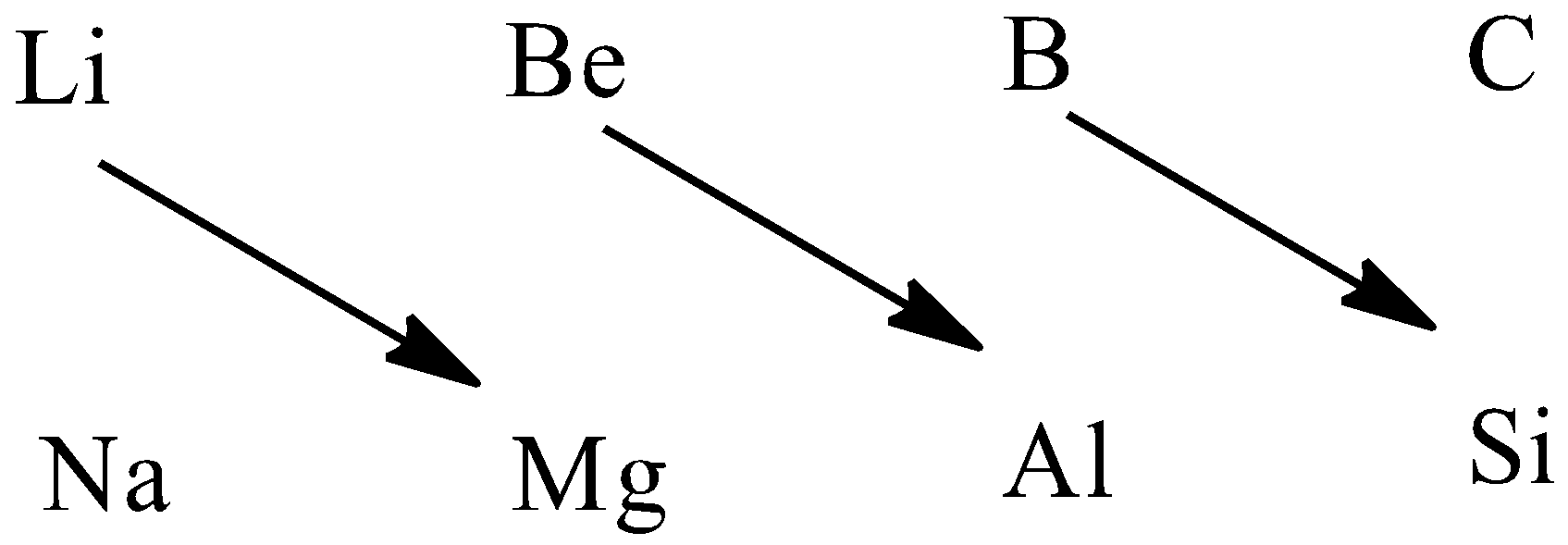
Therefore, the diagonal relationships are $Li,Mg$ , $Be,Al$ , $B,Si$ .
Hence, $C,P$ group of elements does not show a diagonal relationship.
The correct option is (D).
Note:
We have to know that the diagonal relationship is formed due to the identical size of ions. As we move towards the right across a period in the periodic table, then the size of the atom reduces, and when we move downward across a group then the size of the atoms gradually increases.
Complete answer:
We have to know that the above points,
The corner to corner neighbors show a ton of similitudes. Such a relationship is displayed as you move left to right and down the gathering; the occasional table has contradicting factors.
A few connections happen due to the various manners by which numerous nuclear properties shift down gatherings and across times of the Periodic Table.
Notwithstanding the gathering and period connections, the components of s and p-block components likewise show corner to corner connections.
On moving corner to corner across the occasional table, the components show certain likenesses which are anyway undeniably less articulated than the similitudes inside a gathering. The corner to corner relationship is especially perceptible in the components of the second and third times of the intermittent table.

Therefore, the diagonal relationships are $Li,Mg$ , $Be,Al$ , $B,Si$ .
Hence, $C,P$ group of elements does not show a diagonal relationship.
The correct option is (D).
Note:
We have to know that the diagonal relationship is formed due to the identical size of ions. As we move towards the right across a period in the periodic table, then the size of the atom reduces, and when we move downward across a group then the size of the atoms gradually increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




