
Which has maximum dipole moment?
A.
B.
C.

D.





Answer
541.8k+ views
Hint: Dipole moment describes the charge separation of the compound and it is denoted by symbol $\mu $. When the bond angle is given then we can use the formula:
$\mu =\sqrt{{{x}^{2}}+{{y}^{2}}+2xy\cos \theta }$
Complete step-by-step answer:
When a bond is formed between two atoms having a difference in electronegativity, then the electrons of the bond are slightly towards the atom having higher electronegativity. This is described by the dipole moment, which tells the charge separation between the atoms of the bond and it is denoted by the symbol $\mu $. It is shown by an arrow in which the head part is towards the more electronegative atom and the tail part is towards the less electronegative atom.
Now, in the options given in the question, there is the direction of the dipole moment and there is an angle between the atoms. So, we can use the formula:
$\mu =\sqrt{{{x}^{2}}+{{y}^{2}}+2xy\cos \theta }$
We can clearly see that the $\cos \theta $ decides the magnitude of the dipole moment.
In option (a), the angle between the dipole moments is ${{40}^{\circ }}$.
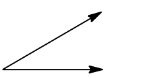
In option (b), the angle between the dipole moments is ${{90}^{\circ }}$.
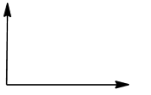
In option (c), the angle between the dipole moments is ${{120}^{\circ }}$.
In option (d), the angle between the dipole moments is ${{180}^{\circ }}$.
The maximum value of $\cos \theta $ will be at ${{0}^{\circ }}$ because the its value is 1, and at ${{90}^{\circ }}$, the value of $\cos \theta $ is 0. Thereafter, on increasing the bond angle, the value will become negative.
Therefore, the lower the bond angle, the higher will be the dipole moment.
Hence the correct answer is an option (a).
Note: When the angle is ${{180}^{\circ }}$, which means one dipole moment is in the opposite direction of the other, then the dipole moment will cancel out each other, and the value will be zero.
$\mu =\sqrt{{{x}^{2}}+{{y}^{2}}+2xy\cos \theta }$
Complete step-by-step answer:
When a bond is formed between two atoms having a difference in electronegativity, then the electrons of the bond are slightly towards the atom having higher electronegativity. This is described by the dipole moment, which tells the charge separation between the atoms of the bond and it is denoted by the symbol $\mu $. It is shown by an arrow in which the head part is towards the more electronegative atom and the tail part is towards the less electronegative atom.
Now, in the options given in the question, there is the direction of the dipole moment and there is an angle between the atoms. So, we can use the formula:
$\mu =\sqrt{{{x}^{2}}+{{y}^{2}}+2xy\cos \theta }$
We can clearly see that the $\cos \theta $ decides the magnitude of the dipole moment.
In option (a), the angle between the dipole moments is ${{40}^{\circ }}$.
In option (b), the angle between the dipole moments is ${{90}^{\circ }}$.
In option (c), the angle between the dipole moments is ${{120}^{\circ }}$.
In option (d), the angle between the dipole moments is ${{180}^{\circ }}$.
The maximum value of $\cos \theta $ will be at ${{0}^{\circ }}$ because the its value is 1, and at ${{90}^{\circ }}$, the value of $\cos \theta $ is 0. Thereafter, on increasing the bond angle, the value will become negative.
Therefore, the lower the bond angle, the higher will be the dipole moment.
Hence the correct answer is an option (a).
Note: When the angle is ${{180}^{\circ }}$, which means one dipole moment is in the opposite direction of the other, then the dipole moment will cancel out each other, and the value will be zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




