
Which is an example of amorphous carbon?
A.Charcoal
B.Coke
C.Lamp black
D.All of the above
Answer
579.6k+ views
Hint: Basically, amorphous carbon is free, reactive carbon that does not have any crystalline structure. True amorphous carbon has localized pi-electrons and its bonds which are formed are inconsistent with other allotropes of carbon.
Complete step by step answer:
Generally, the phenomenon by which an element can exist in more than one physical state is called allotropy. There are two allotropes of carbon namely amorphous carbon allotropes and crystalline carbon allotropes. The allotropes of carbon can be either amorphous or crystalline.
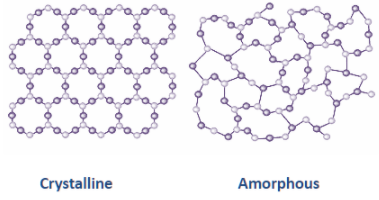
Now, in mineralogy, amorphous carbon is generally used for coal, soot, carbide-derived carbon and other impure forms of carbon that are neither graphite or diamond. Moreover, chemical bonds among atoms are a mixture of $s{p^2}$ and $s{p^3}$ hybridized bonds with a high concentration of bonds. Because the amorphous carbon is thermodynamically in a metastable state and the ratio of these bonds is variable, so the properties of amorphous carbon greatly depend on the formation methods and conditions. In the laboratory, amorphous carbon can be produced by physical vapor deposition, chemical vapor deposition and ion irradiation of diamond or graphite.
Now, all the options are correct as charcoal and coal are forms of amorphous carbon and lamp black is a type of carbon obtained from the soot of burned fat, oil, tar and resin.
Hence, option D is correct.
Note: Diamond is a well-known allotrope of carbon that exhibits hardness and high dispersion of light. The other one is graphene. It is a single layer of carbon atoms arranged in one plane. It is further a material of interest due to its high electron mobility and its application in electronics.
Complete step by step answer:
Generally, the phenomenon by which an element can exist in more than one physical state is called allotropy. There are two allotropes of carbon namely amorphous carbon allotropes and crystalline carbon allotropes. The allotropes of carbon can be either amorphous or crystalline.

Now, in mineralogy, amorphous carbon is generally used for coal, soot, carbide-derived carbon and other impure forms of carbon that are neither graphite or diamond. Moreover, chemical bonds among atoms are a mixture of $s{p^2}$ and $s{p^3}$ hybridized bonds with a high concentration of bonds. Because the amorphous carbon is thermodynamically in a metastable state and the ratio of these bonds is variable, so the properties of amorphous carbon greatly depend on the formation methods and conditions. In the laboratory, amorphous carbon can be produced by physical vapor deposition, chemical vapor deposition and ion irradiation of diamond or graphite.
Now, all the options are correct as charcoal and coal are forms of amorphous carbon and lamp black is a type of carbon obtained from the soot of burned fat, oil, tar and resin.
Hence, option D is correct.
Note: Diamond is a well-known allotrope of carbon that exhibits hardness and high dispersion of light. The other one is graphene. It is a single layer of carbon atoms arranged in one plane. It is further a material of interest due to its high electron mobility and its application in electronics.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




