
Which is incorrect IUPAC name:-
(A)- 3-Pentyne
(B)- 3-Methyl-2-butanone
(C)- 2-Ethyl-3-methyl-1-butene
(D)- 3-Ethyl-2-methyl pentane
Answer
566.7k+ views
Hint: According to the IUPAC, for the correct nomenclature of any molecule always choose the long alkyl chain or long alkyl chain with functional group as main chain and try to provide low numbering to the substituents which are present on the main chain. And also write the name of substituents in the increasing alphabetic manner.
Complete step by step solution: According to the name given in the option (A) i.e. 3-Pentyne, the molecule will be as:
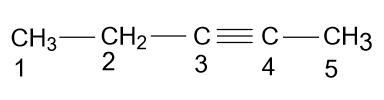
-But it is wrong because according to the rules of IUPAC you have to give low numbering to the substituents or bonds present in the main chain, so according to these rules the name of the above compound should be 2-Pentyne.
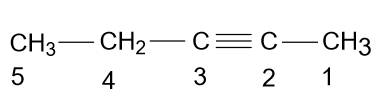
- According to the name which is given in the option (B) i.e. 3-Methyl-2-butanone, the molecule will be as:
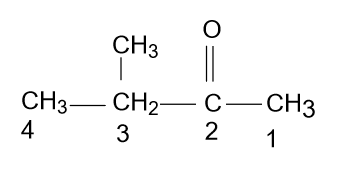
Which is right, because the function group containing the chain is the main chain and also numbering is done correctly.
- According to the name which is given in the option (C) i.e. 2-Ethyl-3-methyl-1-butene, the molecule will be as:
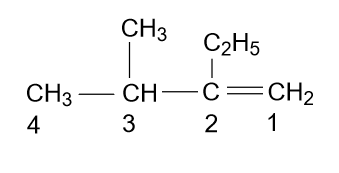
Which is right, because a long chain is the main chain and also numbering is done correctly with alphabetic increasing order.
- According to the name which is given in the option (D) i.e. 3-Ethyl-2-methyl pentane, the molecule will be as:
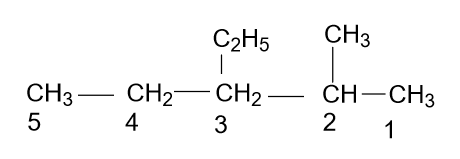
Which is right, because a long chain is the main chain and also numbering is done correctly with alphabetic increasing order.
Note: Here some of you do wrong nomenclature by getting confused in between the substituents, bonds and name of the substituents. So always keep in mind that if a methyl & ethyl group is present, then you should put the name of ethyl first and methyl second which depends on the alphabetic arrangement, not on the size of the substituents.
Complete step by step solution: According to the name given in the option (A) i.e. 3-Pentyne, the molecule will be as:
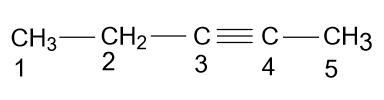
-But it is wrong because according to the rules of IUPAC you have to give low numbering to the substituents or bonds present in the main chain, so according to these rules the name of the above compound should be 2-Pentyne.
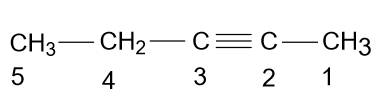
- According to the name which is given in the option (B) i.e. 3-Methyl-2-butanone, the molecule will be as:

Which is right, because the function group containing the chain is the main chain and also numbering is done correctly.
- According to the name which is given in the option (C) i.e. 2-Ethyl-3-methyl-1-butene, the molecule will be as:

Which is right, because a long chain is the main chain and also numbering is done correctly with alphabetic increasing order.
- According to the name which is given in the option (D) i.e. 3-Ethyl-2-methyl pentane, the molecule will be as:

Which is right, because a long chain is the main chain and also numbering is done correctly with alphabetic increasing order.
Note: Here some of you do wrong nomenclature by getting confused in between the substituents, bonds and name of the substituents. So always keep in mind that if a methyl & ethyl group is present, then you should put the name of ethyl first and methyl second which depends on the alphabetic arrangement, not on the size of the substituents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




