
Which is more ionic?
NaF, NaCl, NaBr, NaI
Answer
579.6k+ views
Hint: Ionic compounds are basically defined as the crystalline solids that are formed by neatly packed ions of opposite charge. These are usually formed when metals react with non-metals. They generally break into pieces when pressure is applied and hence are considered as brittle.
Complete step by step answer:
Ionic compounds are the ion compounds. These ions are atoms that gain or lose electrons and thus resulting in a net positive or negative charge. Moreover, the metals tend to lose electrons and have a positive charge and become cations whereas non- metals tend to gain electrons and have a negative charge and become anions.
Now, the ionic compounds include salts, oxides, hydroxides, sulfides and majority of inorganic compounds. Moreover, the ionic solids are held together by the electrostatic attraction between the positive and negative ions. For example, the sodium ions attract chloride ions and the chloride ion attracts the sodium ions. This further results in a three-dimensional structure of sodium and chloride ions. The forces of attraction between the ions hold them in the structures. The structure is as shown:
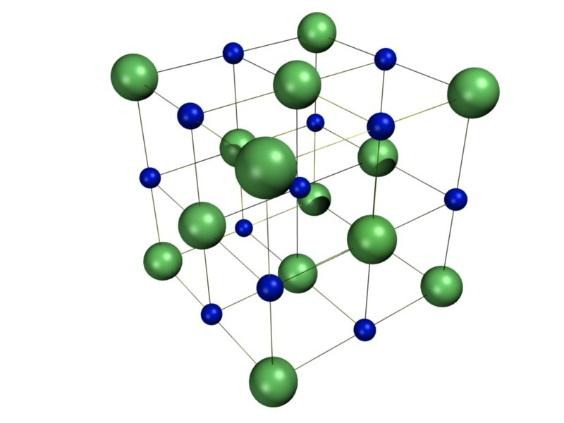
Now, among halogens i.e. chlorine, fluorine, bromine and iodine, fluorine has the highest electronegativity and hence it is considered as the most ionic followed by chlorine and bromine.
Hence, NaF is more ionic.
Note: The ionic compounds have high melting and boiling points and appear to be strong and brittle. They are generally soluble in polar solvents such as water whereas solubility tends to decrease in non-polar solvents such as petrol, gasoline etc. Moreover, ionic compounds do not conduct electricity in the solid state but are good conductors in molten states.
Complete step by step answer:
Ionic compounds are the ion compounds. These ions are atoms that gain or lose electrons and thus resulting in a net positive or negative charge. Moreover, the metals tend to lose electrons and have a positive charge and become cations whereas non- metals tend to gain electrons and have a negative charge and become anions.
Now, the ionic compounds include salts, oxides, hydroxides, sulfides and majority of inorganic compounds. Moreover, the ionic solids are held together by the electrostatic attraction between the positive and negative ions. For example, the sodium ions attract chloride ions and the chloride ion attracts the sodium ions. This further results in a three-dimensional structure of sodium and chloride ions. The forces of attraction between the ions hold them in the structures. The structure is as shown:

Now, among halogens i.e. chlorine, fluorine, bromine and iodine, fluorine has the highest electronegativity and hence it is considered as the most ionic followed by chlorine and bromine.
Hence, NaF is more ionic.
Note: The ionic compounds have high melting and boiling points and appear to be strong and brittle. They are generally soluble in polar solvents such as water whereas solubility tends to decrease in non-polar solvents such as petrol, gasoline etc. Moreover, ionic compounds do not conduct electricity in the solid state but are good conductors in molten states.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




