
Which is the correct order of increasing energy of the listed orbitals in the atom of titanium? (At. No. 22)
(A) 3s 3p 3d 4s
(B) 3s 3p 4s 3d
(C) 3s 4s 3p 3d
(D) 4s 3s 3p 3d
Answer
579.9k+ views
Hint: Write the electronic configuration of titanium. Recollect the order in which filling of electrons in the orbitals takes place. The question is asking us to write the increasing order of energies of the orbitals of titanium atoms. Think about the principle or rule which governs the way in which electronic configuration is written.
Complete step by step solution:
- Titanium is a d-block element in the periodic table. It has atomic number 22. Its electronic configuration is given as,
\[{}^{22}Ti=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{2}}\]
- The filling of electrons in the orbitals is in accordance with Aufbau’s rule.
- According to Aufbau’s rule, the electrons will start filling in orbitals having lowest energy and then move on to orbitals having higher energy. Aufbau’s rule states that the electrons will be filled in orbitals according to their increasing order of energies.
- Aufbau’s rule is represented as,
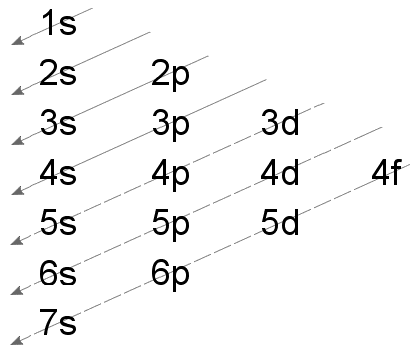
- The order of energy of different orbitals in an atom is given below,
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
- Therefore, the correct order of increasing energy of the listed orbitals in the atom of titanium is 3s 3p 4s 3d.
- Therefore, the correct answer is option (B).
Note: Remember the electrons are filled up in the orbitals in increasing order of their energies. The principle which tells us about the sequence in which electrons will get filled up in the orbitals is known as Aufbau’s principle. 1s orbital has the lowest energy.
Complete step by step solution:
- Titanium is a d-block element in the periodic table. It has atomic number 22. Its electronic configuration is given as,
\[{}^{22}Ti=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{2}}\]
- The filling of electrons in the orbitals is in accordance with Aufbau’s rule.
- According to Aufbau’s rule, the electrons will start filling in orbitals having lowest energy and then move on to orbitals having higher energy. Aufbau’s rule states that the electrons will be filled in orbitals according to their increasing order of energies.
- Aufbau’s rule is represented as,

- The order of energy of different orbitals in an atom is given below,
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
- Therefore, the correct order of increasing energy of the listed orbitals in the atom of titanium is 3s 3p 4s 3d.
- Therefore, the correct answer is option (B).
Note: Remember the electrons are filled up in the orbitals in increasing order of their energies. The principle which tells us about the sequence in which electrons will get filled up in the orbitals is known as Aufbau’s principle. 1s orbital has the lowest energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




