
Which is the least reactive metal in the reactivity series?
A-Copper
B-Mercury
C-Silver
D-Gold
Answer
597k+ views
Hint: The arrangement of metals in decreasing order of their reactivity is called a Reactivity series. This series is used to determine whether given metal can displace another metal in a displacement reaction.
Complete step by step answer:
We know that reactivity series is arranged in such a way that as we move from bottom to top, reactivity of the metals increases.
The series is given as –
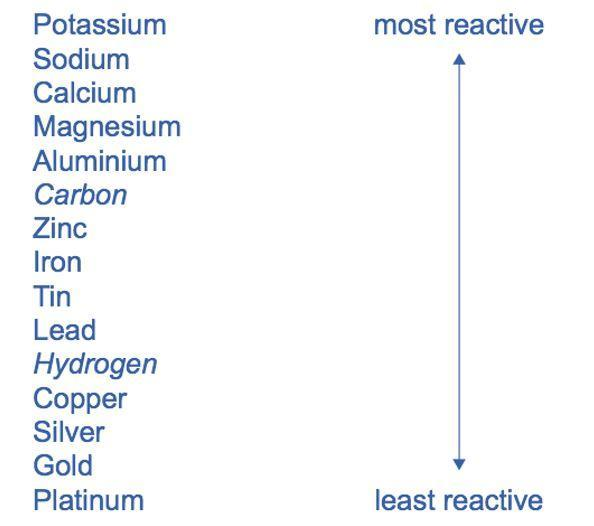
As we can see in the series, out of all the given options gold is the least reactive metal.
So, option D is correct.
Additional information:
Along with reactivity the reducing nature of the metals also goes on increasing as we move from top to bottom. This means that the metals at the top of the reactivity series are most powerful reducing agents. The electropositive nature of the elements also increases as we move from bottom to top in the series. The metals placed on the higher side also tend to tarnish or corrode easily.
All metals which are placed above Hydrogen in the series release hydrogen gas on reacting with dilute hydrochloric acid or dilute sulphuric acid.
Suppose we are given a displacement reaction and we have been told to determine which metal will displace the given metal. Then, if we look into the reactivity series, the metal which is placed above the given metal will displace it in the reaction.
Note: As we move down in the reactivity series, electron donating tendency of the metals decreases. So, the metals at the very bottom are least reactive and the ones at the top of the series are highly reactive.
Complete step by step answer:
We know that reactivity series is arranged in such a way that as we move from bottom to top, reactivity of the metals increases.
The series is given as –

As we can see in the series, out of all the given options gold is the least reactive metal.
So, option D is correct.
Additional information:
Along with reactivity the reducing nature of the metals also goes on increasing as we move from top to bottom. This means that the metals at the top of the reactivity series are most powerful reducing agents. The electropositive nature of the elements also increases as we move from bottom to top in the series. The metals placed on the higher side also tend to tarnish or corrode easily.
All metals which are placed above Hydrogen in the series release hydrogen gas on reacting with dilute hydrochloric acid or dilute sulphuric acid.
Suppose we are given a displacement reaction and we have been told to determine which metal will displace the given metal. Then, if we look into the reactivity series, the metal which is placed above the given metal will displace it in the reaction.
Note: As we move down in the reactivity series, electron donating tendency of the metals decreases. So, the metals at the very bottom are least reactive and the ones at the top of the series are highly reactive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




