
Which is the weakest acid here?
(A) sulphuric acid
(B) Hydrochloric acid
(C) citric acid
(D) None of the above
Answer
565.2k+ views
Hint: Consider the functional groups present in the given acids. Determine if the given acids are organic or inorganic in nature. Inorganic acids are stronger than organic acids.
Complete answer:
The dissociation power of an acid determines its strength. Strong acids completely dissociate in aqueous solution whereas weak acids dissociate to a small extent. If you compare 1M solution of a strong acid with 1M solution of a weak acid, you will find that the hydrogen ion concentration for 1M strong acid solution is greater than the hydrogen ion concentration for 1M solution of weak acid. A strong acid solution has lower pH than a weak acid solution of the same concentration.
Write the structures of sulphuric acid, hydrochloric acid and citric acid as shown below:
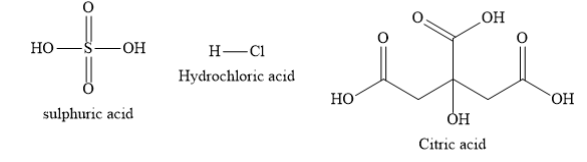
Citric acid is the organic acid containing carboxylic functional groups. Sulphuric acid and hydrochloric acid are inorganic acids. Organic carboxylic acids are usually weaker acids than inorganic acids. Citric acid is the weakest acid among the given acids. Sulphuric acid and hydrochloric acid are strong acids.
Hence, the correct option is the option (C).
Note: pH is the negative logarithm to base 10 of hydrogen ion concentration. A neutral solution has pH of 7. An acidic solution has pH less than 7 and a basic solution has pH more than 7.
Complete answer:
The dissociation power of an acid determines its strength. Strong acids completely dissociate in aqueous solution whereas weak acids dissociate to a small extent. If you compare 1M solution of a strong acid with 1M solution of a weak acid, you will find that the hydrogen ion concentration for 1M strong acid solution is greater than the hydrogen ion concentration for 1M solution of weak acid. A strong acid solution has lower pH than a weak acid solution of the same concentration.
Write the structures of sulphuric acid, hydrochloric acid and citric acid as shown below:

Citric acid is the organic acid containing carboxylic functional groups. Sulphuric acid and hydrochloric acid are inorganic acids. Organic carboxylic acids are usually weaker acids than inorganic acids. Citric acid is the weakest acid among the given acids. Sulphuric acid and hydrochloric acid are strong acids.
Hence, the correct option is the option (C).
Note: pH is the negative logarithm to base 10 of hydrogen ion concentration. A neutral solution has pH of 7. An acidic solution has pH less than 7 and a basic solution has pH more than 7.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




