
Which molecule has a bent shape?
Answer
522.3k+ views
Hint : If molecules have a non-collinear arrangement of two adjacent bonds, then the resultant geometry of the molecule is bent. It is also called angular or V- shape. Water is a molecule having bent shape. There are two hydrogen atoms and one oxygen atom in one water molecule.
Complete Step By Step Answer:
Water molecule is a V-shaped molecule. Water has a bent shape resulting from tetrahedral electron pair geometry. Water is polar and it is a universal solvent. It has high heat of vaporization and also high heat of capacity. Water is less dense in solid form than in its liquid form. It has cohesive and adhesive properties. The oxygen in water molecules has six valence electrons and needs two more electrons to achieve octet configuration. Hydrogen atoms give two electrons to oxygen and help in completing octets. The structure of water molecule is given below:
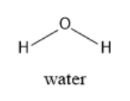
Therefore, water molecules have a bent shape.
Note :
In a water molecule, two hydrogen atoms are bound to a central oxygen atom by a pair of electrons that are shared between them. Two electrons out of six outer shell electrons of oxygen are used for this purpose, leaving behind four electrons into two nonbonding pairs. The water molecule is expected to have tetrahedral geometry in which the angle between electron pairs is ${104^ \circ }$. But, because of the two nonbonding electron pairs on oxygen atoms, these undergo strong repulsion. Due to this reason, the shape of the water molecule is bent.
Complete Step By Step Answer:
Water molecule is a V-shaped molecule. Water has a bent shape resulting from tetrahedral electron pair geometry. Water is polar and it is a universal solvent. It has high heat of vaporization and also high heat of capacity. Water is less dense in solid form than in its liquid form. It has cohesive and adhesive properties. The oxygen in water molecules has six valence electrons and needs two more electrons to achieve octet configuration. Hydrogen atoms give two electrons to oxygen and help in completing octets. The structure of water molecule is given below:

Therefore, water molecules have a bent shape.
Note :
In a water molecule, two hydrogen atoms are bound to a central oxygen atom by a pair of electrons that are shared between them. Two electrons out of six outer shell electrons of oxygen are used for this purpose, leaving behind four electrons into two nonbonding pairs. The water molecule is expected to have tetrahedral geometry in which the angle between electron pairs is ${104^ \circ }$. But, because of the two nonbonding electron pairs on oxygen atoms, these undergo strong repulsion. Due to this reason, the shape of the water molecule is bent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




