
Which molecule has a trigonal planar geometry?
A. $I{F_3}$
B. $PC{l_3}$
C. $N{H_3}$
D. $B{F_3}$
Answer
584.4k+ views
Hint: The molecular geometry of a molecule gives the general shape, bend angles, Torsional angles, bond lengths etc. the Propertius like polarity, reactivity, Colour, state of matter etc. can be determined by knouting the molecular geometry of the molecule.
Complete step by step answer: The molecular geometry is determined with the help of some special techniques named as spectroscopic and diffraction methods, example: $X - Ray,$$NMR,\;1R$, Raman spectroscopy etc.
The geometry of individual molecules is discussed below:-
- $I{F_3}$
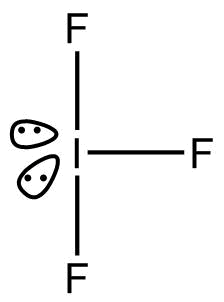
According to ${\text{VSEPR}}$, Theory, geometry of $I{F_3}$is trigonal bi pyramidal.
-$PC{l_3}$
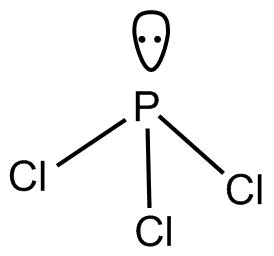
According to $VSEPR$Theory geometry of $PC{l_3}$ is trigonal pyramidal.
-$N{H_3}$
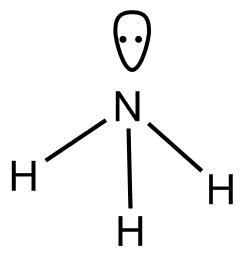
According to $VSEPR$Theory geometry of $N{H_3}$ is trigonal pyramidal.
- $B{F_3}$
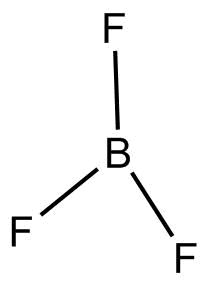
According to $VSEPR$Theory geometry of $B{F_3}$ is trigonal planar.
$B{F_3}$ or Boron trifluoride is an organic compound with a pungent smell, it is a colorless, poisonous gas.
$B{F_3}$ does not have any lone pair, the three $F$atoms are arranged around central Boron at a bond angle of ${120^{o} }$and bond length of ${130^{o} }$.
Many other molecules such as formaldehyde $\left( {C{H_2}O} \right),$sulfur trioxide $\left( {S{O_3}} \right)$, phosgene $\left( {COC{l_2}} \right),$ozone $\left( {{O_3}} \right)$etc. have similar trigonal planar geometry.
Hence, the correct option is $\left( D \right)B{F_{3.}}$
Note: $VSEPR$ is a short form of the valence shell electron pair repulsion theory. This theory. Can predict the geometry of many molecules, especially those belonging to the p – block, in $VSEPR$ theory, the geometry of a molecule atom. The geometry of a molecule depends upon the number of valence shell electrons around the central atom.
The pairs of electrons try to occupy minimum repulsion and maximal distance orientation in space.
Complete step by step answer: The molecular geometry is determined with the help of some special techniques named as spectroscopic and diffraction methods, example: $X - Ray,$$NMR,\;1R$, Raman spectroscopy etc.
The geometry of individual molecules is discussed below:-
- $I{F_3}$

According to ${\text{VSEPR}}$, Theory, geometry of $I{F_3}$is trigonal bi pyramidal.
-$PC{l_3}$

According to $VSEPR$Theory geometry of $PC{l_3}$ is trigonal pyramidal.
-$N{H_3}$

According to $VSEPR$Theory geometry of $N{H_3}$ is trigonal pyramidal.
- $B{F_3}$

According to $VSEPR$Theory geometry of $B{F_3}$ is trigonal planar.
$B{F_3}$ or Boron trifluoride is an organic compound with a pungent smell, it is a colorless, poisonous gas.
$B{F_3}$ does not have any lone pair, the three $F$atoms are arranged around central Boron at a bond angle of ${120^{o} }$and bond length of ${130^{o} }$.
Many other molecules such as formaldehyde $\left( {C{H_2}O} \right),$sulfur trioxide $\left( {S{O_3}} \right)$, phosgene $\left( {COC{l_2}} \right),$ozone $\left( {{O_3}} \right)$etc. have similar trigonal planar geometry.
Hence, the correct option is $\left( D \right)B{F_{3.}}$
Note: $VSEPR$ is a short form of the valence shell electron pair repulsion theory. This theory. Can predict the geometry of many molecules, especially those belonging to the p – block, in $VSEPR$ theory, the geometry of a molecule atom. The geometry of a molecule depends upon the number of valence shell electrons around the central atom.
The pairs of electrons try to occupy minimum repulsion and maximal distance orientation in space.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




