
Which of the following are heterocyclic aromatic compounds?
This question has multiple correct options
(A)
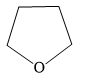
B)
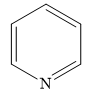
C)
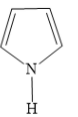
D)
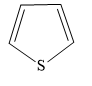




Answer
578.4k+ views
Hint: First determine which of the given heterocyclic compounds are planar. Then find out which of these compounds contains the conjugate system of \[\left( {4n + 2} \right)\pi \] electrons.
Complete step by step answer:
You can classify organic compounds in two major classes, cyclic and non-cyclic compounds. You can further classify cyclic compounds into homocyclic and heterocyclic compounds. You can further classify heterocyclic aromatic compounds into aliphatic heterocyclic compounds and aromatic heterocyclic compounds.
Cyclic compounds contain one or more rings. Usually the rings containing five or six carbon atoms are stable. In homocyclic compounds, the ring contains only one type of atoms, usually carbon atoms. In heterocyclic compounds, apart from carbon atoms, there is at least one other atom such as nitrogen, oxygen or sulphur atom.
Aliphatic compounds do not obey Huckel’s rule. Aromatic compounds follow Huckel’s rule.
According to Huckel's rule, a cyclic, planar molecule is aromatic, when it has a conjugated system of \[\left( {4n + 2} \right)\pi \] electrons.
Tetrahydrofuran is a heterocyclic compound. But it is not an aromatic compound. Although it contains a lone pair of electrons, these electrons are not delocalized as there is no conjugated system.
In pyridine, there are 3 carbon-carbon double bonds that form conjugated \[\left( {4n + 2} \right)\pi = \left( {4\left( 1 \right) + 2} \right)\pi = 6\pi \] electron system. The lone pairs of electrons on nitrogen atoms are not considered.
In 1H-pyrrole, there are 2 carbon-carbon double bonds that form conjugated \[\left( {4n + 2} \right)\pi = \left( {4\left( 1 \right) + 2} \right)\pi = 6\pi \] electron system. This includes one lone pair of electrons on a nitrogen atom.
In thiophene, there are 2 carbon-carbon double bonds that form conjugated \[\left( {4n + 2} \right)\pi = \left( {4\left( 1 \right) + 2} \right)\pi = 6\pi \] electron system. This includes one lone pair of electrons on a sulphur atom.
The compounds pyridine, 1H-pyrrole and thiophene are heterocyclic aromatic compounds.
Hence, the correct options are the options (B), (C) and (D).
Note: There are some compounds that are planar and have a conjugated system of \[4n\pi \] electrons. Such compounds are called antiaromatic compounds. The third class of compounds is non aromatic compounds. This class includes all compounds except aromatic compounds and antiaromatic compounds.
Complete step by step answer:
You can classify organic compounds in two major classes, cyclic and non-cyclic compounds. You can further classify cyclic compounds into homocyclic and heterocyclic compounds. You can further classify heterocyclic aromatic compounds into aliphatic heterocyclic compounds and aromatic heterocyclic compounds.
Cyclic compounds contain one or more rings. Usually the rings containing five or six carbon atoms are stable. In homocyclic compounds, the ring contains only one type of atoms, usually carbon atoms. In heterocyclic compounds, apart from carbon atoms, there is at least one other atom such as nitrogen, oxygen or sulphur atom.
Aliphatic compounds do not obey Huckel’s rule. Aromatic compounds follow Huckel’s rule.
According to Huckel's rule, a cyclic, planar molecule is aromatic, when it has a conjugated system of \[\left( {4n + 2} \right)\pi \] electrons.
Tetrahydrofuran is a heterocyclic compound. But it is not an aromatic compound. Although it contains a lone pair of electrons, these electrons are not delocalized as there is no conjugated system.
In pyridine, there are 3 carbon-carbon double bonds that form conjugated \[\left( {4n + 2} \right)\pi = \left( {4\left( 1 \right) + 2} \right)\pi = 6\pi \] electron system. The lone pairs of electrons on nitrogen atoms are not considered.
In 1H-pyrrole, there are 2 carbon-carbon double bonds that form conjugated \[\left( {4n + 2} \right)\pi = \left( {4\left( 1 \right) + 2} \right)\pi = 6\pi \] electron system. This includes one lone pair of electrons on a nitrogen atom.
In thiophene, there are 2 carbon-carbon double bonds that form conjugated \[\left( {4n + 2} \right)\pi = \left( {4\left( 1 \right) + 2} \right)\pi = 6\pi \] electron system. This includes one lone pair of electrons on a sulphur atom.
The compounds pyridine, 1H-pyrrole and thiophene are heterocyclic aromatic compounds.
Hence, the correct options are the options (B), (C) and (D).
Note: There are some compounds that are planar and have a conjugated system of \[4n\pi \] electrons. Such compounds are called antiaromatic compounds. The third class of compounds is non aromatic compounds. This class includes all compounds except aromatic compounds and antiaromatic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




