
Which of the following are uses of Sulphur?
(A) Sulphur is used in preparation of conducting wires
(B) A mixture of zinc and Sulphur on heating with Sulphur produces a greyish reduce of zinc sulfide
(C) Sulphur is an element belonging to VIA group
(D) Sulphur is a nonmetallic element
Answer
583.5k+ views
Hint: Sulphur is non-metal, interlace and order less. It is yellow crystalline solid. It occurs as a sulfate and sulfites and sulfates minerals.
Complete step by step answer:
As the uses of Sulphur asked in question so we first discuss the importance of Sulphur.
Sulphur is an essential element for life. It is a minor constituent of fats, body fluids and statute minerals. Sulphur is a key component in most proteins and also present in amino acids.
The given question has multiple connect options.
(B) A mixture of zinc and Sulphur on heating with Sulphur produces a greyish residue of zinc Sulfide.
(C) Sulphur is an element belonging to VI A group.
(D) Sulphur is non-metallic element
Therefore, from the above explanation the correct option is (B) A mixture of zinc and Sulphur on heating with Sulphur produces a greyish reduce of zinc sulfide, (C) Sulphur is an element belonging to VIA group and (D) Sulphur is a nonmetallic element.
Additional Information:
Sulphur is a chemical element with symbol S and atomic number 16.
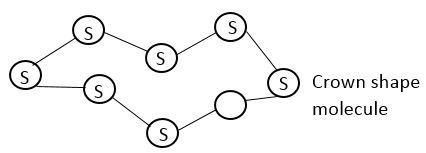
Under normal conditions Sulphur atoms form cyclic octa atomic molecule with chemical formula S. It forms crown shape structure.
Electronic configuration of Sulphur is, $[Ne]3{s^2}3{p^4}$
Sulphur can also be spelled as sulfur.
Sulphur is used in Vulcanization of black Rubber, as fungicide and in black grey powder.
Most Sulphur is used in production of sulphuric acid.
Sulphuric acid is the most important chemical.
Note:
Sulphur is a non-metallic plate yellow solid. These are many allotropes of Sulphur like Rhombic, amorphous and Prismatic.
Sulphur is odorless and insoluble in water.
Many substances containing Sulphur have distinct odors.
Complete step by step answer:
As the uses of Sulphur asked in question so we first discuss the importance of Sulphur.
Sulphur is an essential element for life. It is a minor constituent of fats, body fluids and statute minerals. Sulphur is a key component in most proteins and also present in amino acids.
The given question has multiple connect options.
(B) A mixture of zinc and Sulphur on heating with Sulphur produces a greyish residue of zinc Sulfide.
(C) Sulphur is an element belonging to VI A group.
(D) Sulphur is non-metallic element
Therefore, from the above explanation the correct option is (B) A mixture of zinc and Sulphur on heating with Sulphur produces a greyish reduce of zinc sulfide, (C) Sulphur is an element belonging to VIA group and (D) Sulphur is a nonmetallic element.
Additional Information:
Sulphur is a chemical element with symbol S and atomic number 16.
Under normal conditions Sulphur atoms form cyclic octa atomic molecule with chemical formula S. It forms crown shape structure.

Electronic configuration of Sulphur is, $[Ne]3{s^2}3{p^4}$
Sulphur can also be spelled as sulfur.
Sulphur is used in Vulcanization of black Rubber, as fungicide and in black grey powder.
Most Sulphur is used in production of sulphuric acid.
Sulphuric acid is the most important chemical.
Note:
Sulphur is a non-metallic plate yellow solid. These are many allotropes of Sulphur like Rhombic, amorphous and Prismatic.
Sulphur is odorless and insoluble in water.
Many substances containing Sulphur have distinct odors.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




