
Which of the following biphenyl is optically active?
(A)
(B)
(C)
(D)




Answer
550.5k+ views
Hint: Optical activity is shown by compounds in which plane of symmetry is not present, if plane of symmetry is present in any compound then they will never show optical activity.
Complete answer:
Optical activity is a kind of property of any compound which rotates the plane polarized light to the right or left side from the direction of upcoming light.
In biphenyls two benzene rings are present and if a plane of symmetry is present within the molecule then they don't show optical activity.
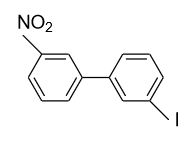
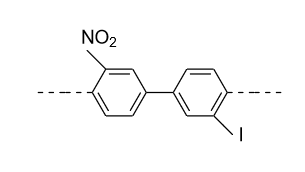
- In option (A), in the 1ST ring nitro (${\text{N}}{{\text{O}}_{\text{2}}}$) group is present in the meta (m) position and in the 2nd ring iodine (${\text{I}}$) is present in the meta’ (m’) position so plane of symmetry is present in this biphenyl in the following manner and it doesn’t show optical activity.
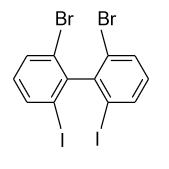
- In option (B), in the ortho positions of both the rings bromine (${\text{Br}}$) atoms are present and in the ortho’ positions iodine (${\text{I}}$) atoms are present. Due to presence of substitution in both the rings at ortho position strain is produced and the compound will not present no more planar in structure. So, there is no plane of symmetry existing and the compound will not show optical activity.
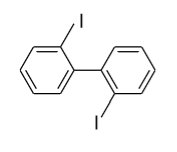
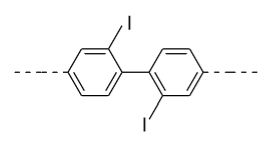
- In option (C), in the 1ST ring iodine (${\text{I}}$) group is present in the ortho (o) position and in the 2nd ring iodine (${\text{I}}$) is present in the ortho’ (o’) position so plane of symmetry is present in this biphenyl in the following manner and it doesn’t show optical activity.
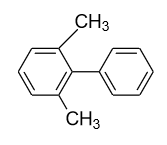
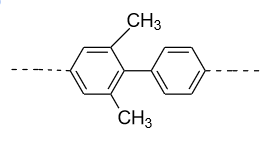
- In option (D), in the 1ST ring methyl group (${\text{C}}{{\text{H}}_{\text{3}}}$) group is present in the ortho (o) and ortho’ (o’) position. So, a plane of symmetry is present in this biphenyl in the following manner and it doesn’t show optical activity.
Hence, option (B) is correct.
Note:
Here some of you may get confused on getting the planarity or non – planarity of the molecule. And for rejecting this type of confusion you should know about the nature of the substituted species at different positions.
Complete answer:
Optical activity is a kind of property of any compound which rotates the plane polarized light to the right or left side from the direction of upcoming light.
In biphenyls two benzene rings are present and if a plane of symmetry is present within the molecule then they don't show optical activity.
- In option (A), in the 1ST ring nitro (${\text{N}}{{\text{O}}_{\text{2}}}$) group is present in the meta (m) position and in the 2nd ring iodine (${\text{I}}$) is present in the meta’ (m’) position so plane of symmetry is present in this biphenyl in the following manner and it doesn’t show optical activity.

- In option (B), in the ortho positions of both the rings bromine (${\text{Br}}$) atoms are present and in the ortho’ positions iodine (${\text{I}}$) atoms are present. Due to presence of substitution in both the rings at ortho position strain is produced and the compound will not present no more planar in structure. So, there is no plane of symmetry existing and the compound will not show optical activity.
- In option (C), in the 1ST ring iodine (${\text{I}}$) group is present in the ortho (o) position and in the 2nd ring iodine (${\text{I}}$) is present in the ortho’ (o’) position so plane of symmetry is present in this biphenyl in the following manner and it doesn’t show optical activity.

- In option (D), in the 1ST ring methyl group (${\text{C}}{{\text{H}}_{\text{3}}}$) group is present in the ortho (o) and ortho’ (o’) position. So, a plane of symmetry is present in this biphenyl in the following manner and it doesn’t show optical activity.

Hence, option (B) is correct.
Note:
Here some of you may get confused on getting the planarity or non – planarity of the molecule. And for rejecting this type of confusion you should know about the nature of the substituted species at different positions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




