
Which of the following can be used in the Friedel Crafts reaction to generate electrophile? This question has multiple correct options
A.
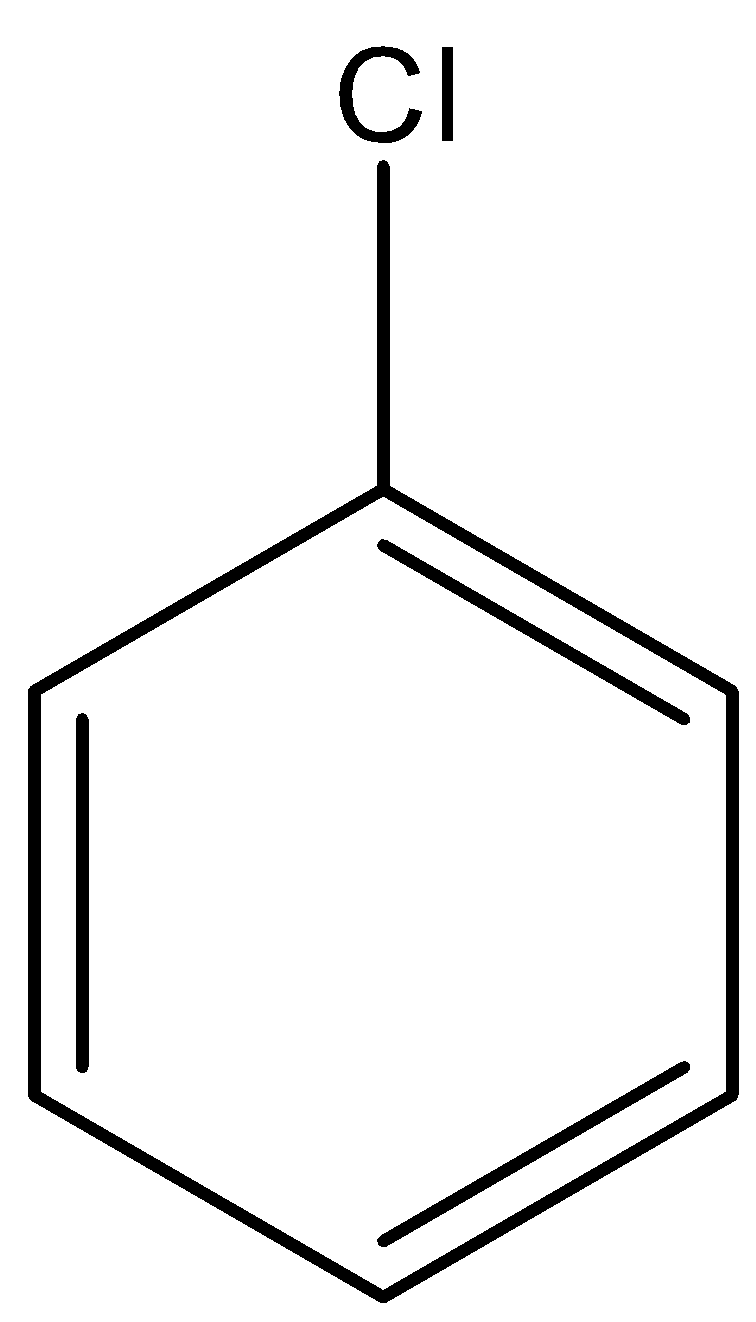
B. \[C{H_2} = CH - Cl\]
C. \[\;C{H_3}C{H_2}CI\] sss
D. \[C{H_2} = CH - C{H_2} - CI\]

Answer
579.9k+ views
Hint: To convert benzene to ethylbenzene there is a very well-known reaction which is the Friedel-Craft alkylation reaction. In presence of aq. \[AlC{l_3}\] it reduces the nucleophilicity of the benzene ring by the formation of the cation of the benzene ring. When the acyl group is substituted into the benzene ring that reaction is also known as Friedel-Crafts acylation of Benzene.
Complete step by step answer:
Friedel-Craft reaction with aryl and vinyl halide is not possible, because the lone pair of halogen undergoes conjugation with the benzene ring and double bond respectively, and forms a partial double bond. Due to this reason, only alkyl and vinyl halide cannot be used as a halide component in a Friedel-craft reaction.
So only alkyl halides are used in Friedel Crafts reaction to generate electrophile. That the halogen group attaches with \[s{p^3}\] hybridized carbon can be used in Friedel Crafts reaction to generate electrophile.
So, the correct options are, C and D.
Additional information:
\[C{H_3}COCl\] is the most reactive substance that contains an acyl group. It is commonly known as acyl chloride or acid chloride.
The benzene ring is reacted with a mixture of ethanoyl chloride \[C{H_3}COCl\] and \[AlC{l_3}\] (aluminum chloride) that acts as a catalyst. The product formed is a ketone called phenylethane.
The reaction is as follows:
\[{C_6}{H_6} + C{H_3}COCl \to {C_6}{H_5}COCl + HCl\]
Or
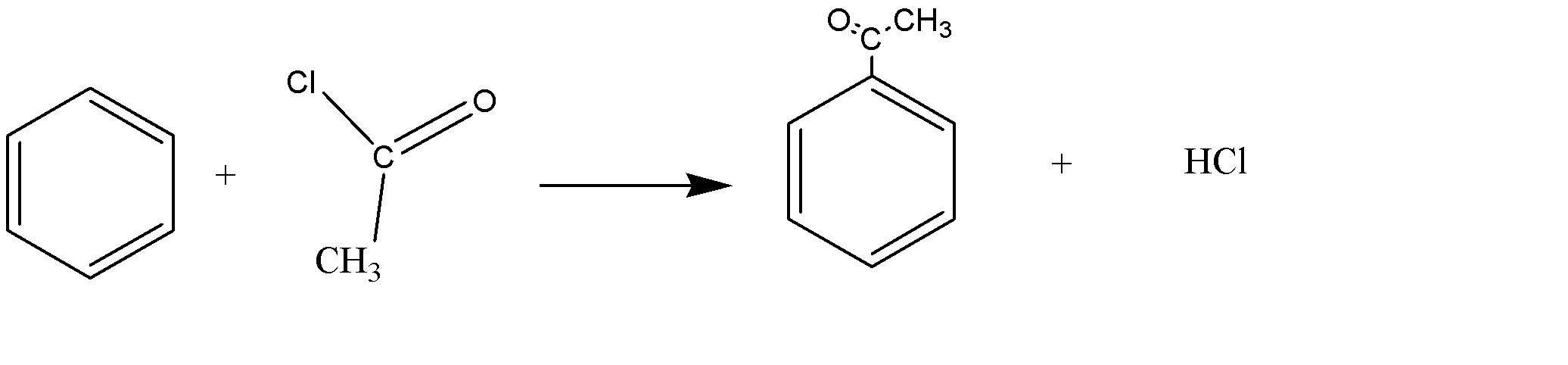
This reaction is an example of an electrophilic substitution reaction. The electrophile is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^{\text{ + }}}\] that is produced by the reaction of ethanoyl chloride and aluminum chloride.
The reaction is as follows:
\[C{H_3}COCl + AlC{l_3} \to C{H_3}C{O^ + } + AlC{l_4}^ - \]
The electrophilic substitution mechanism is divided into 2 stages:
Stage 1 –
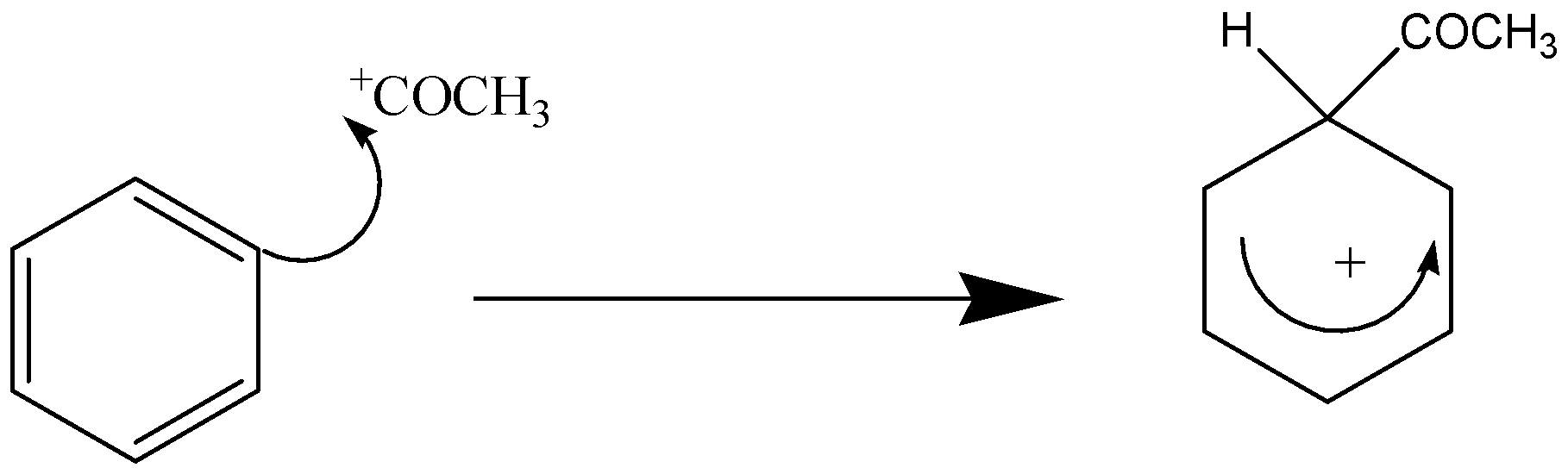
Stage 2 –
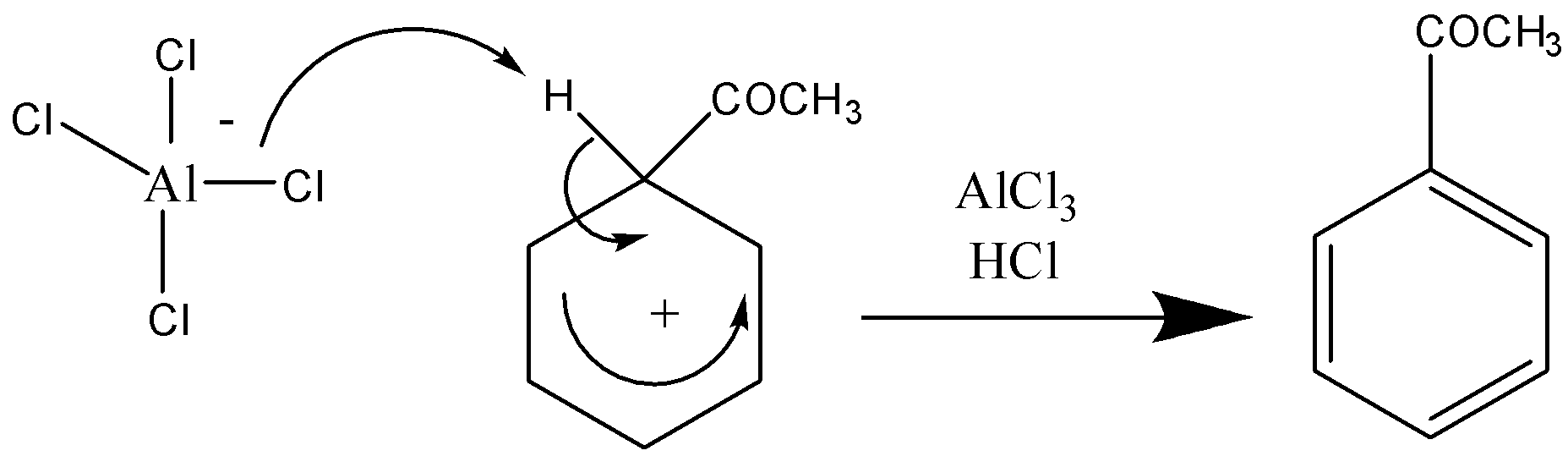
Note:A student can also get confused between electrophilic substitution and nucleophilic substitution. Electrophilic substitution involves the displacement of a functional group by an electrophile, i.e., hydrogen whereas nucleophilic substitution involves the attack of a positively charged atom by a nucleophile.
Complete step by step answer:
Friedel-Craft reaction with aryl and vinyl halide is not possible, because the lone pair of halogen undergoes conjugation with the benzene ring and double bond respectively, and forms a partial double bond. Due to this reason, only alkyl and vinyl halide cannot be used as a halide component in a Friedel-craft reaction.
So only alkyl halides are used in Friedel Crafts reaction to generate electrophile. That the halogen group attaches with \[s{p^3}\] hybridized carbon can be used in Friedel Crafts reaction to generate electrophile.
So, the correct options are, C and D.
Additional information:
\[C{H_3}COCl\] is the most reactive substance that contains an acyl group. It is commonly known as acyl chloride or acid chloride.
The benzene ring is reacted with a mixture of ethanoyl chloride \[C{H_3}COCl\] and \[AlC{l_3}\] (aluminum chloride) that acts as a catalyst. The product formed is a ketone called phenylethane.
The reaction is as follows:
\[{C_6}{H_6} + C{H_3}COCl \to {C_6}{H_5}COCl + HCl\]
Or

This reaction is an example of an electrophilic substitution reaction. The electrophile is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^{\text{ + }}}\] that is produced by the reaction of ethanoyl chloride and aluminum chloride.
The reaction is as follows:
\[C{H_3}COCl + AlC{l_3} \to C{H_3}C{O^ + } + AlC{l_4}^ - \]
The electrophilic substitution mechanism is divided into 2 stages:
Stage 1 –

Stage 2 –

Note:A student can also get confused between electrophilic substitution and nucleophilic substitution. Electrophilic substitution involves the displacement of a functional group by an electrophile, i.e., hydrogen whereas nucleophilic substitution involves the attack of a positively charged atom by a nucleophile.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




