
Which of the following compounds are gem-dihalides?
A.Ethylidene chloride
B.Ethylene dichloride
C.Methylene chloride
D.Benzyl chloride
Answer
577.8k+ views
Hint: Geminal or Gem dihalides are those compounds in which halides are substituted on the same carbon atom. In other words, they have 1,1 relationships. The term ‘hominal’ can also be used instead of gem-dihalides.
Complete step by step answer:
Geminal dihalides are the one where the two halide groups are located on the same carbon atom maintaining a 1,1 relationship.
Ethylidene chloride is also known as 1,1 Dichloroethane. It has 2 Chlorine atoms on the same carbon atom thus has 1,1 relationship. Thus, it is geminal dihalide.
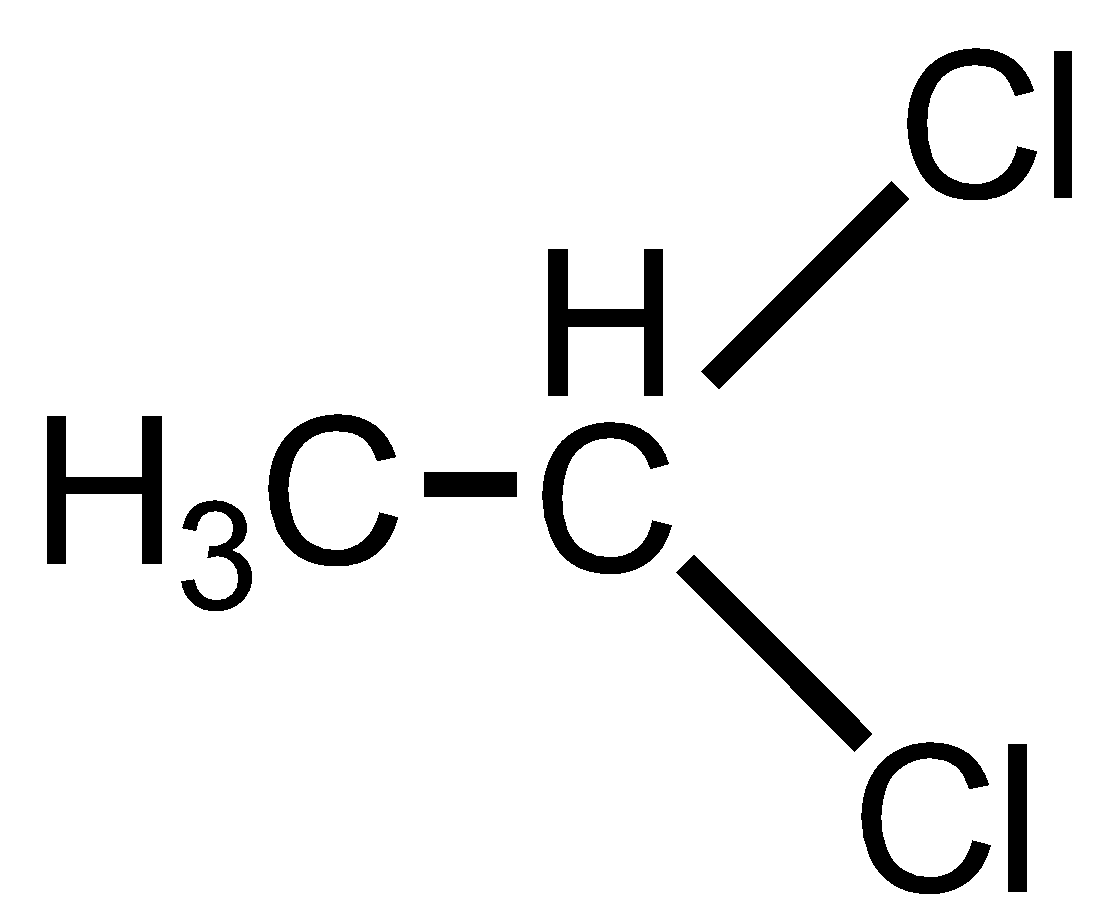
Ethylene dichloride is also known as 1,2 Dichloroethane. It has 2 Chlorine atoms on the different carbon atoms that are adjacent carbon atoms having a 1,2 relationship. Thus, it is not a geminal dihalide.
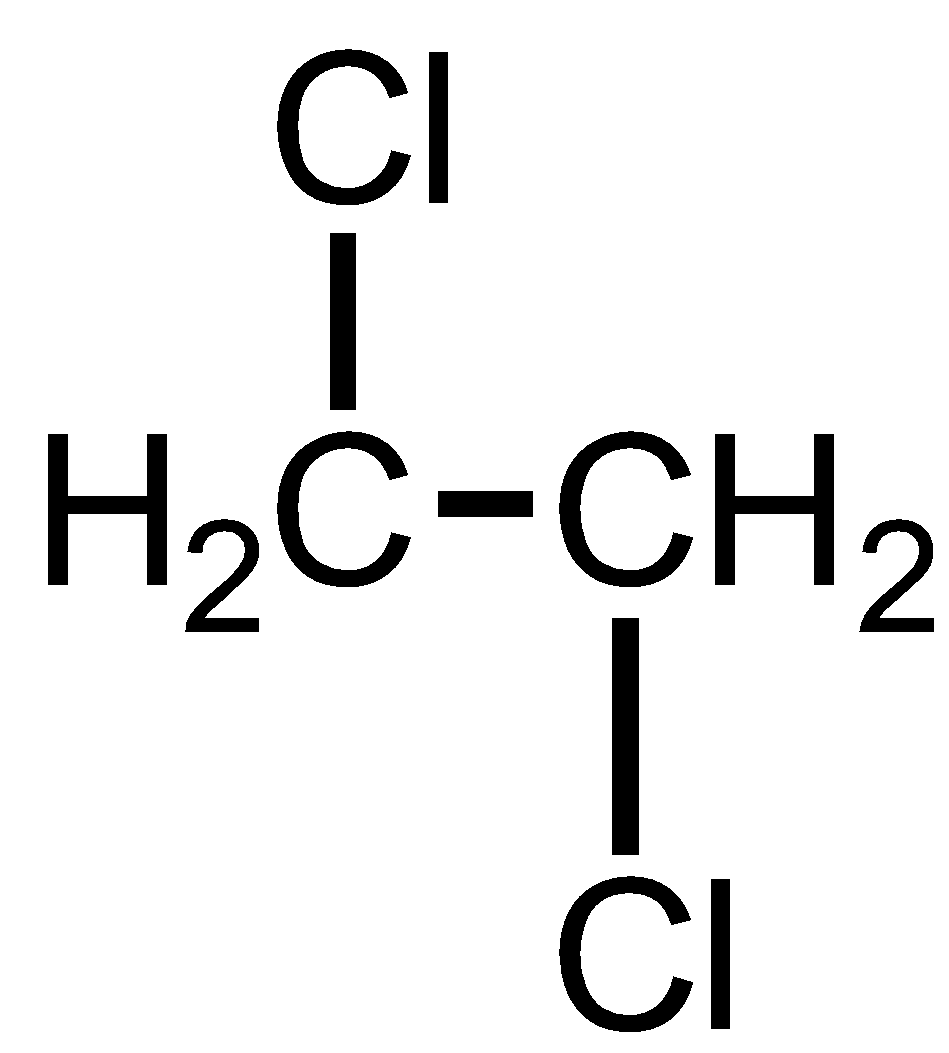
Methylene chloride is also known as 1,1 Dichloro methane. It has 2 Chlorine atoms on the same carbon atom thus has 1,1 relationship. Thus, it is geminal dihalide.
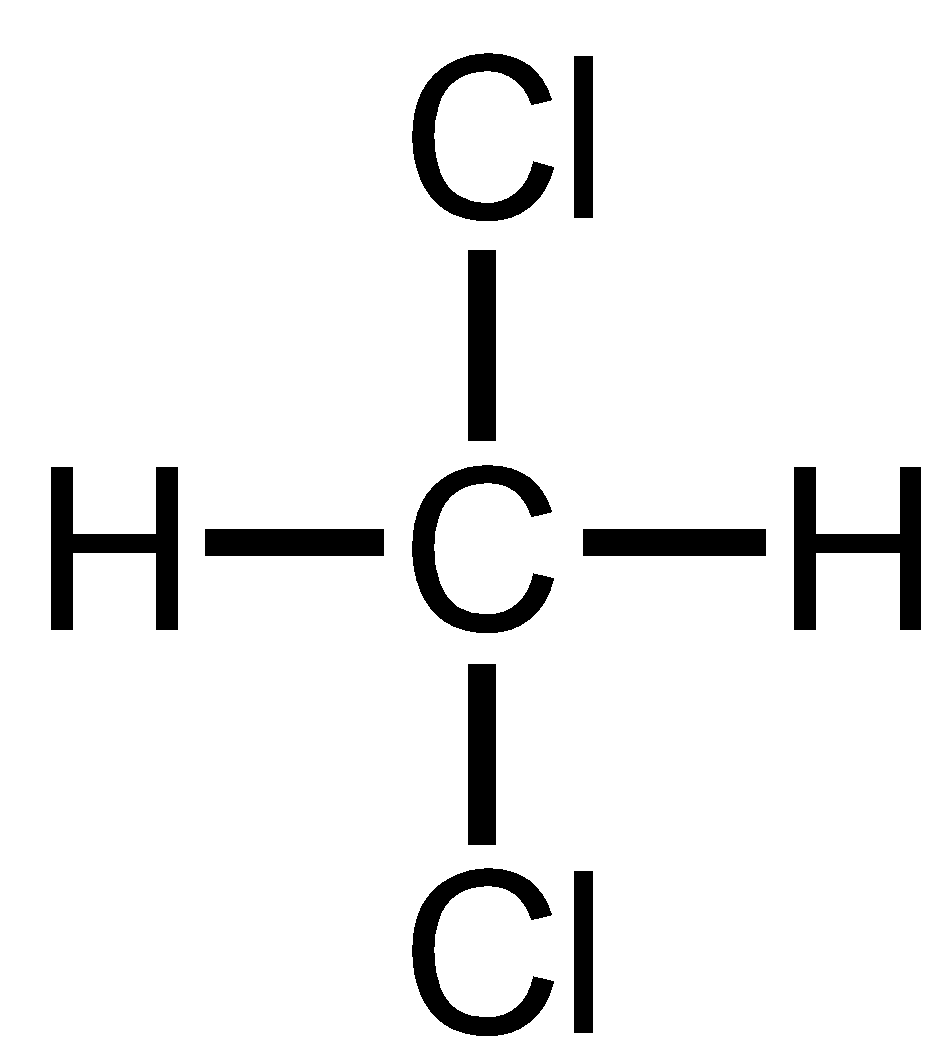
Benzyl chloride has only one chloride atom so there are no dihalides thus ‘gem’ term cannot be prefixed.
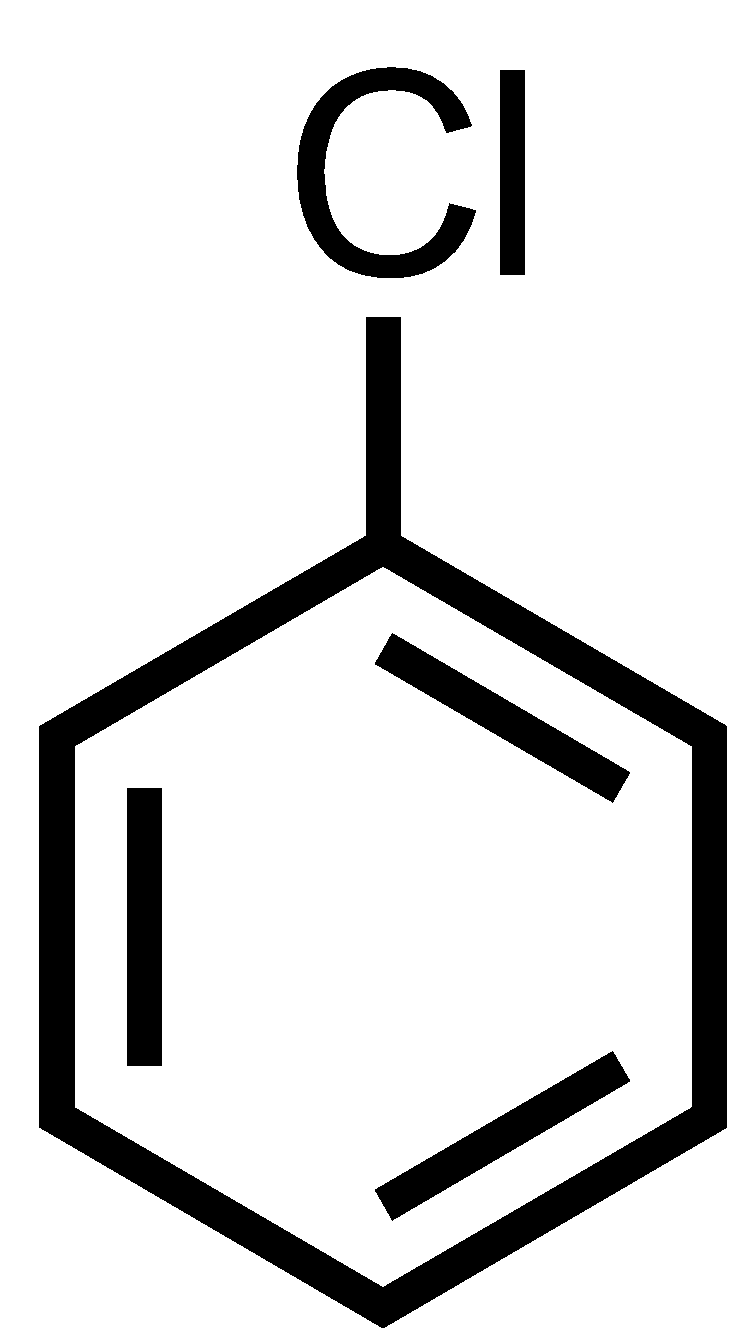
So, the correct options are A and C.
Note:
The other common prefix used for mentioning the substituents is “vicinal”. The term “vicinal dihalides” is used for compounds containing two halides on the adjacent carbon atoms. It has 1,2 relationships.

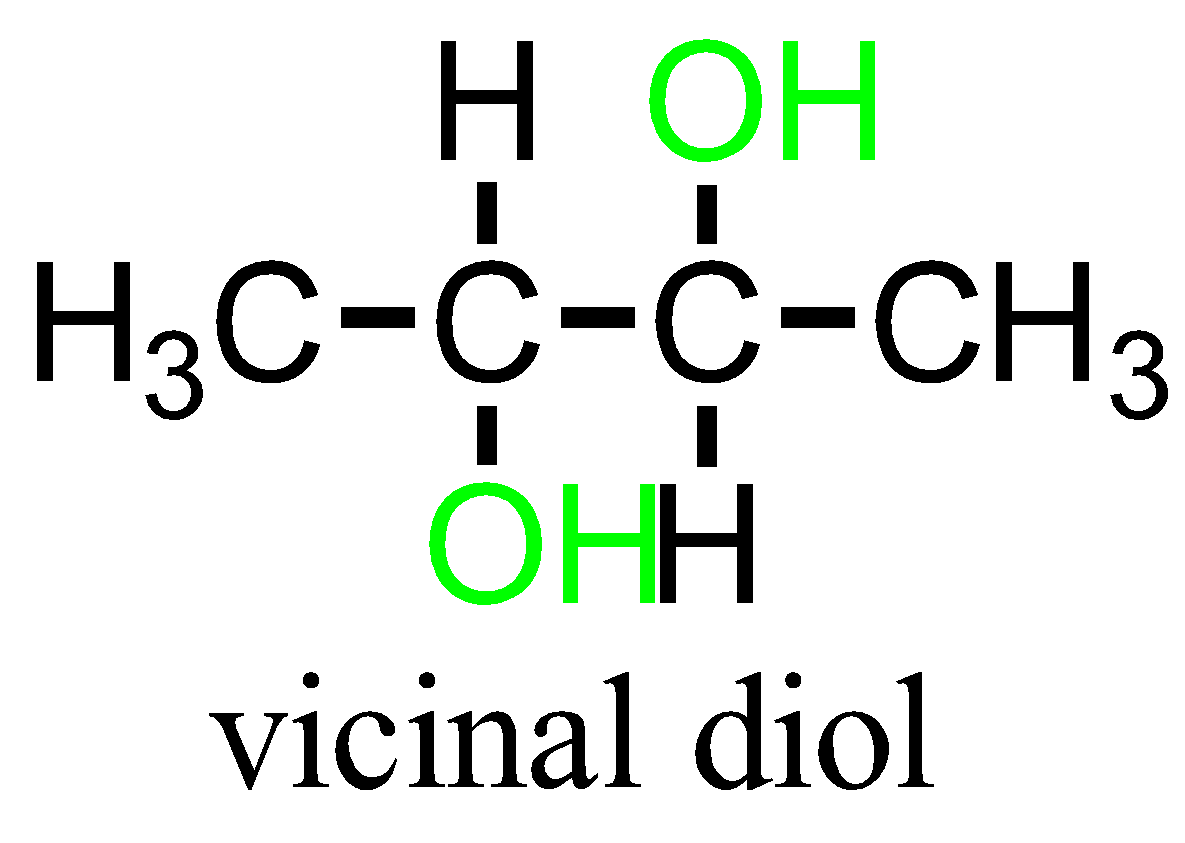
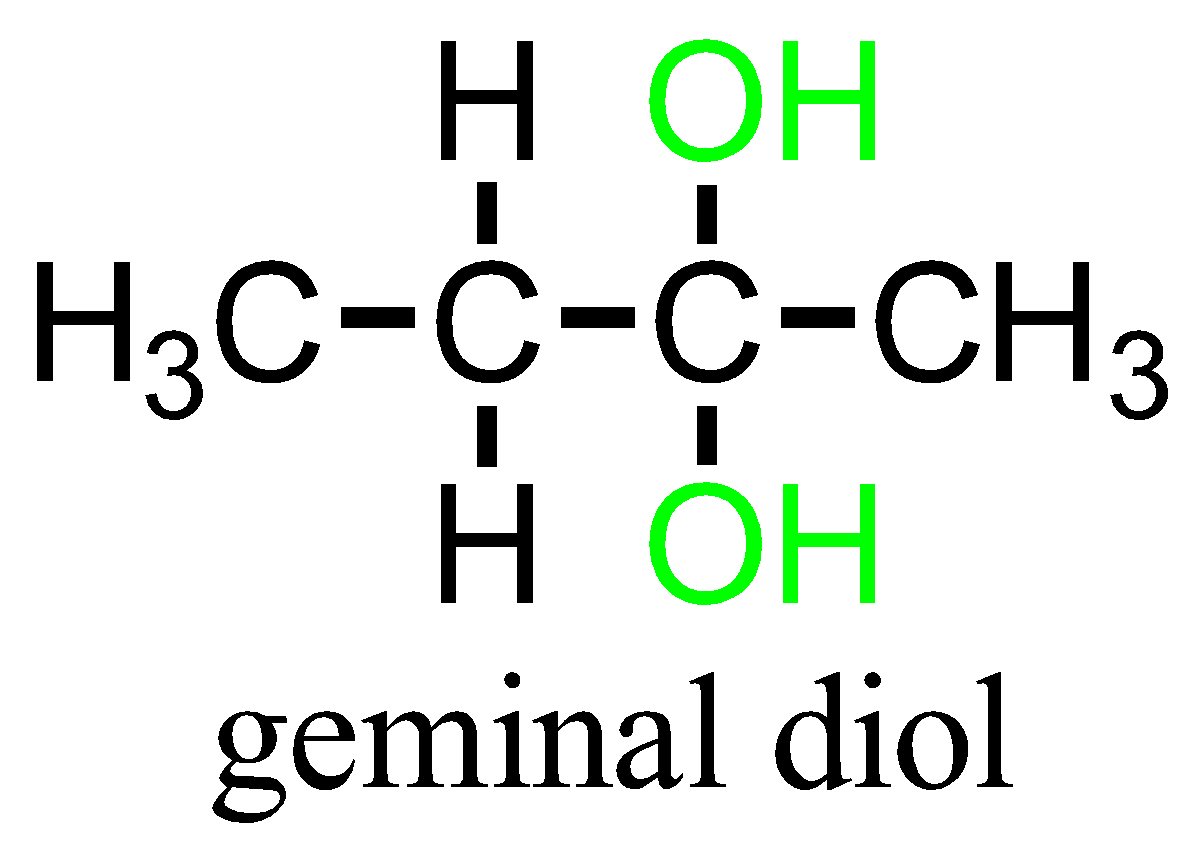
The term “vicinal” and “geminal” can be applied to any substituents that repeat twice. Example: hydroxy groups namely Diols containing compounds.
Complete step by step answer:
Geminal dihalides are the one where the two halide groups are located on the same carbon atom maintaining a 1,1 relationship.
Ethylidene chloride is also known as 1,1 Dichloroethane. It has 2 Chlorine atoms on the same carbon atom thus has 1,1 relationship. Thus, it is geminal dihalide.

Ethylene dichloride is also known as 1,2 Dichloroethane. It has 2 Chlorine atoms on the different carbon atoms that are adjacent carbon atoms having a 1,2 relationship. Thus, it is not a geminal dihalide.

Methylene chloride is also known as 1,1 Dichloro methane. It has 2 Chlorine atoms on the same carbon atom thus has 1,1 relationship. Thus, it is geminal dihalide.

Benzyl chloride has only one chloride atom so there are no dihalides thus ‘gem’ term cannot be prefixed.

So, the correct options are A and C.
Note:
The other common prefix used for mentioning the substituents is “vicinal”. The term “vicinal dihalides” is used for compounds containing two halides on the adjacent carbon atoms. It has 1,2 relationships.

The term “vicinal” and “geminal” can be applied to any substituents that repeat twice. Example: hydroxy groups namely Diols containing compounds.


Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




