
Which of the following compounds do not have all C-C bonds of same length?
(a)
(b)
(c)
(d)




Answer
513.6k+ views
Hint: We need to know that a carbon-carbon bond is a covalent connection between two carbon particles. The most well-known structure is the single bond: a bond made out of two electrons, one from every one of the two molecules. The carbon-carbon single bond is a sigma bond and is shaped between one hybridized orbital from every one of the carbon atoms.
Complete answer:
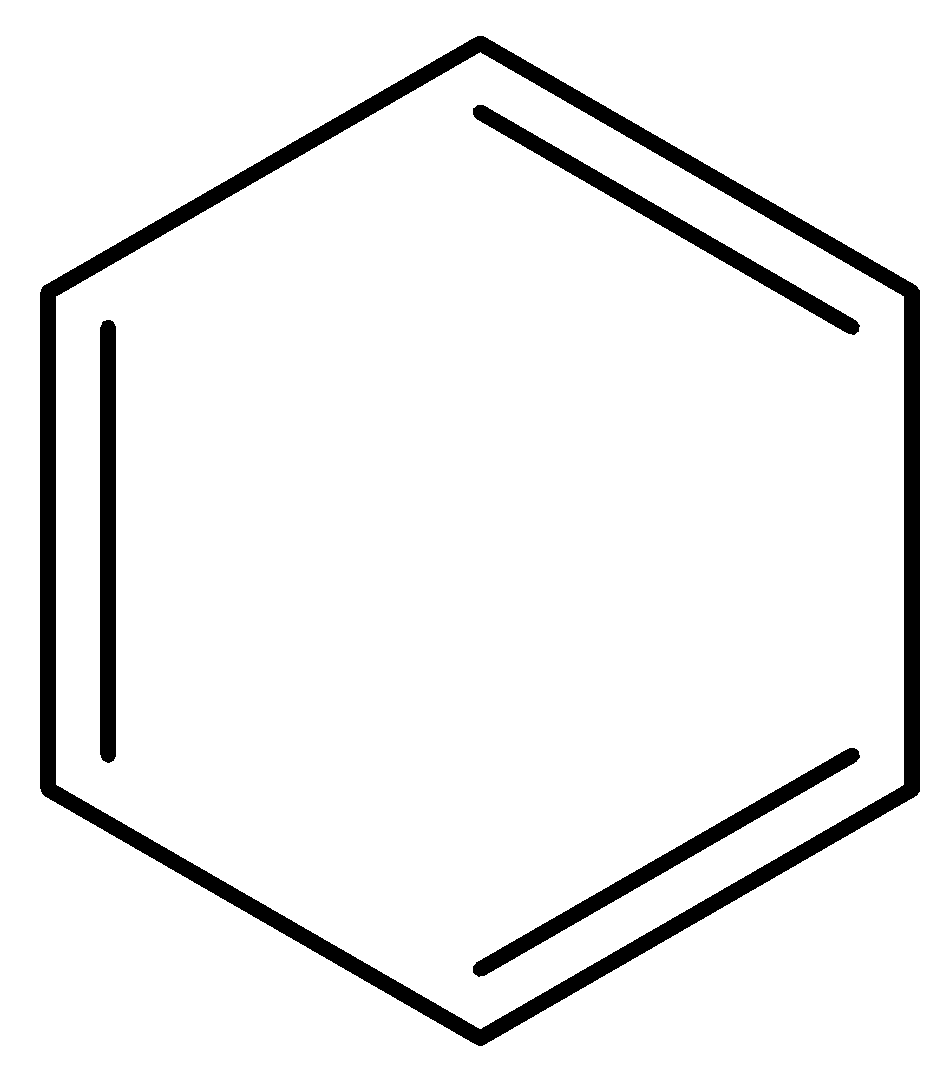
For benzene,
In benzene carbon-carbon bond length between all carbons are equivalent due to reverberation. The pi electrons in benzene are delocalized over all the six carbon atoms. Hence, all the carbon-carbon bond orders in benzene are equivalent.
Therefore, option (a) is incorrect.
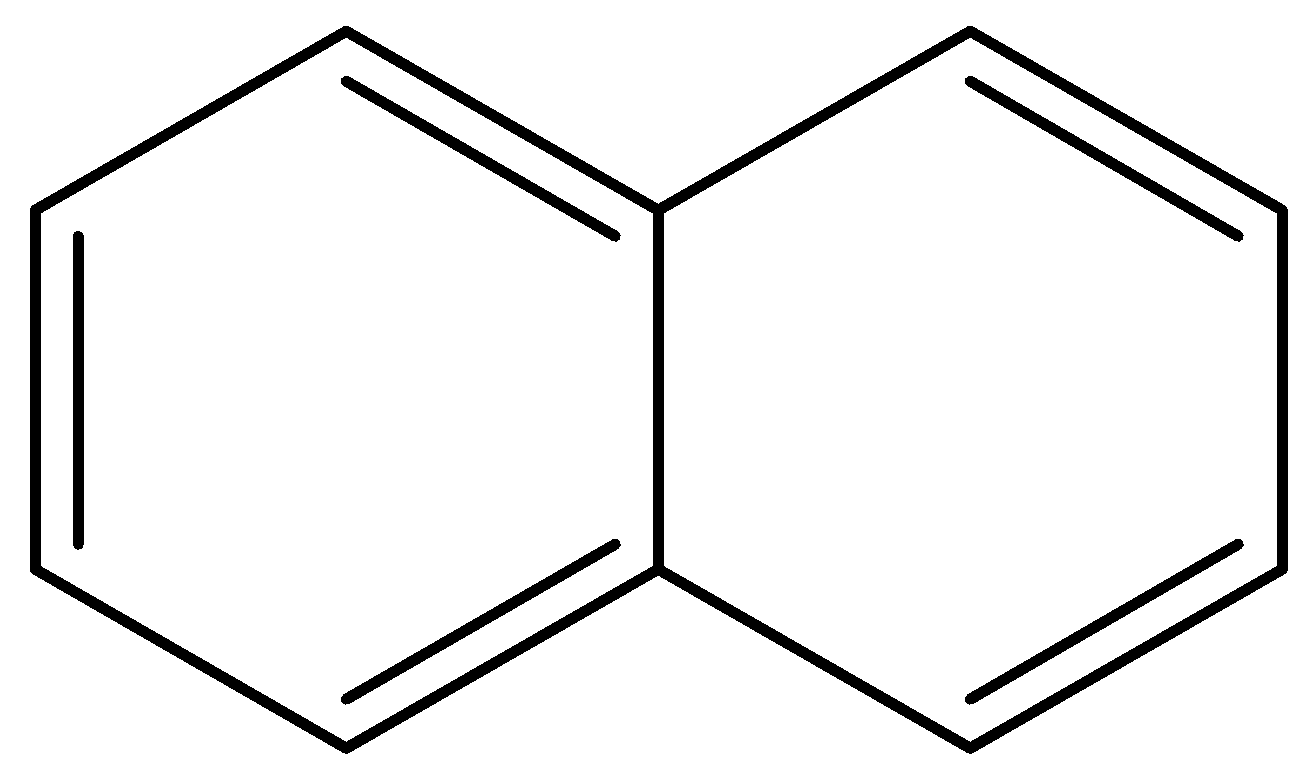
For naphthalene,
At the point when benzene rings strengthen to shape a bicyclic compound as in naphthalene, circles at the intersection re-hybridize under steric strain, restricting the bond and electron thickness. Thus, the bond one and bond two have higher bond request and less antibonding character. Bond three not influenced by strain and having low bond request and higher bond length. Bond four unhindered by the bond strain which is having less strain because of inconsistent bond lengths one, two, and three.
Therefore, option (b) is correct.
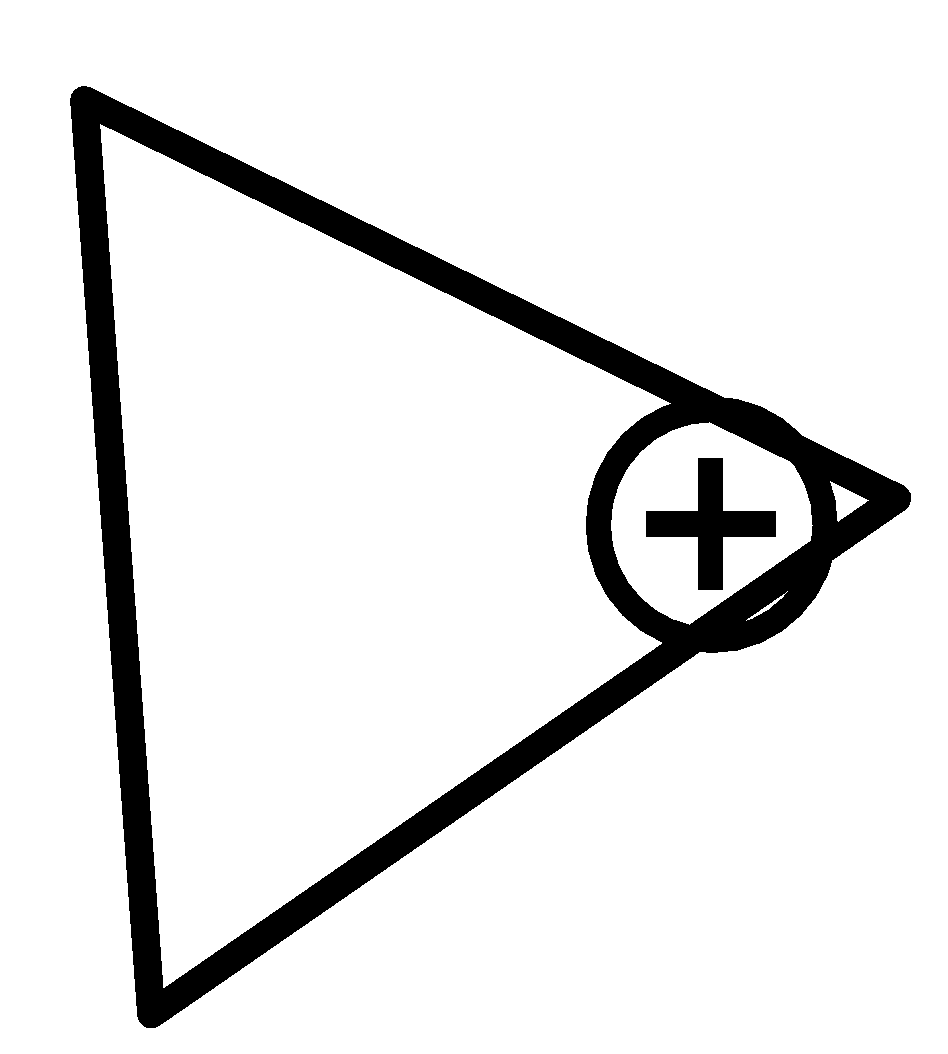
For $cyclopropan - 1 - ylium$ ,
One surprising outcome of twisted holding is that the carbon-carbon bonds in cyclopropane are more fragile than ordinary. Therefore, the bond length of carbon-carbon bonds is equal.
Therefore, option (c) is incorrect.
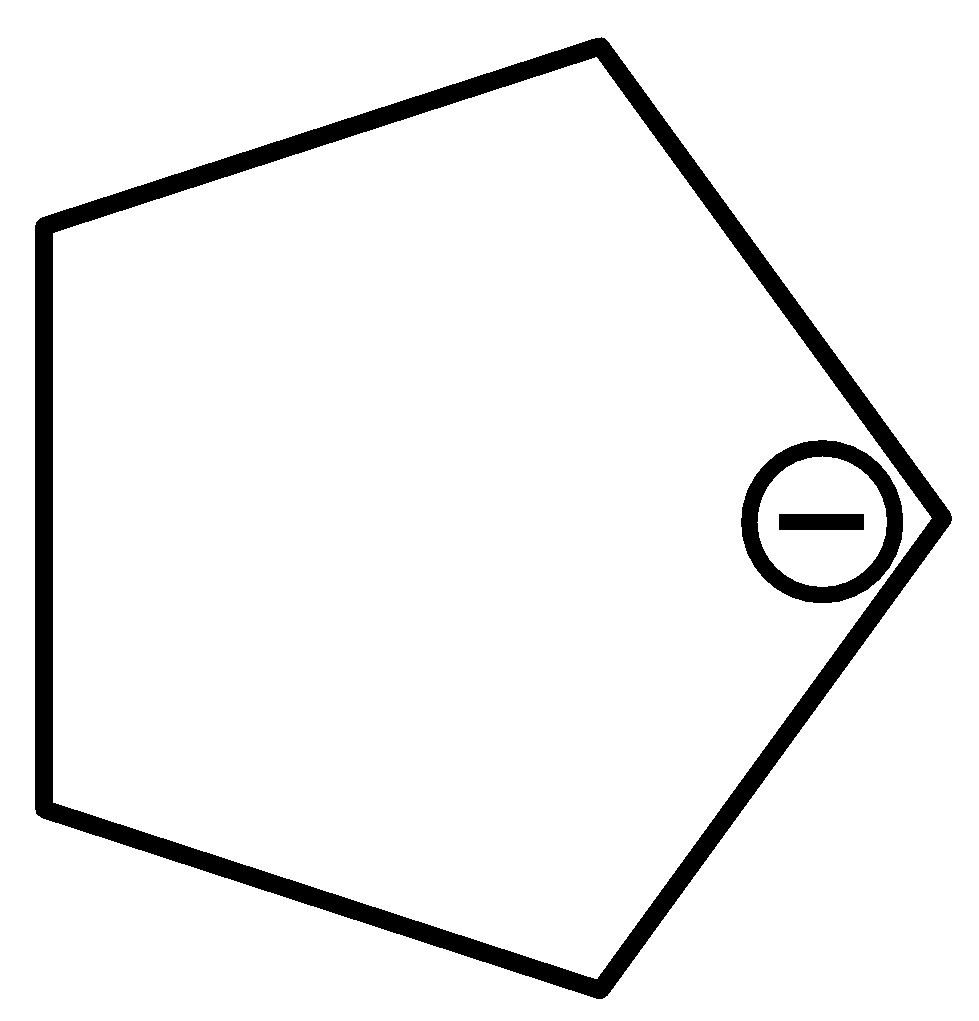
For $cyclopen\tan - 1 - ide$ ,
The bond length of carbon-carbon bonds of the given compound is the same.
Therefore, option (d) is incorrect.
Note:
We must have to know that the length of the bond is controlled by the quantity of reinforced electrons (the bond request). The higher the bond request, the more grounded the draw between the two particles and the more limited the bond length. By and large, the length of the connection between two molecules is roughly the amount of the covalent radii of the two particles.
Complete answer:
For benzene,
In benzene carbon-carbon bond length between all carbons are equivalent due to reverberation. The pi electrons in benzene are delocalized over all the six carbon atoms. Hence, all the carbon-carbon bond orders in benzene are equivalent.
Therefore, option (a) is incorrect.
For naphthalene,
At the point when benzene rings strengthen to shape a bicyclic compound as in naphthalene, circles at the intersection re-hybridize under steric strain, restricting the bond and electron thickness. Thus, the bond one and bond two have higher bond request and less antibonding character. Bond three not influenced by strain and having low bond request and higher bond length. Bond four unhindered by the bond strain which is having less strain because of inconsistent bond lengths one, two, and three.
Therefore, option (b) is correct.
For $cyclopropan - 1 - ylium$ ,
One surprising outcome of twisted holding is that the carbon-carbon bonds in cyclopropane are more fragile than ordinary. Therefore, the bond length of carbon-carbon bonds is equal.
Therefore, option (c) is incorrect.
For $cyclopen\tan - 1 - ide$ ,
The bond length of carbon-carbon bonds of the given compound is the same.
Therefore, option (d) is incorrect.
Note:
We must have to know that the length of the bond is controlled by the quantity of reinforced electrons (the bond request). The higher the bond request, the more grounded the draw between the two particles and the more limited the bond length. By and large, the length of the connection between two molecules is roughly the amount of the covalent radii of the two particles.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




