
Which of the following compounds have delocalized electrons?
A.
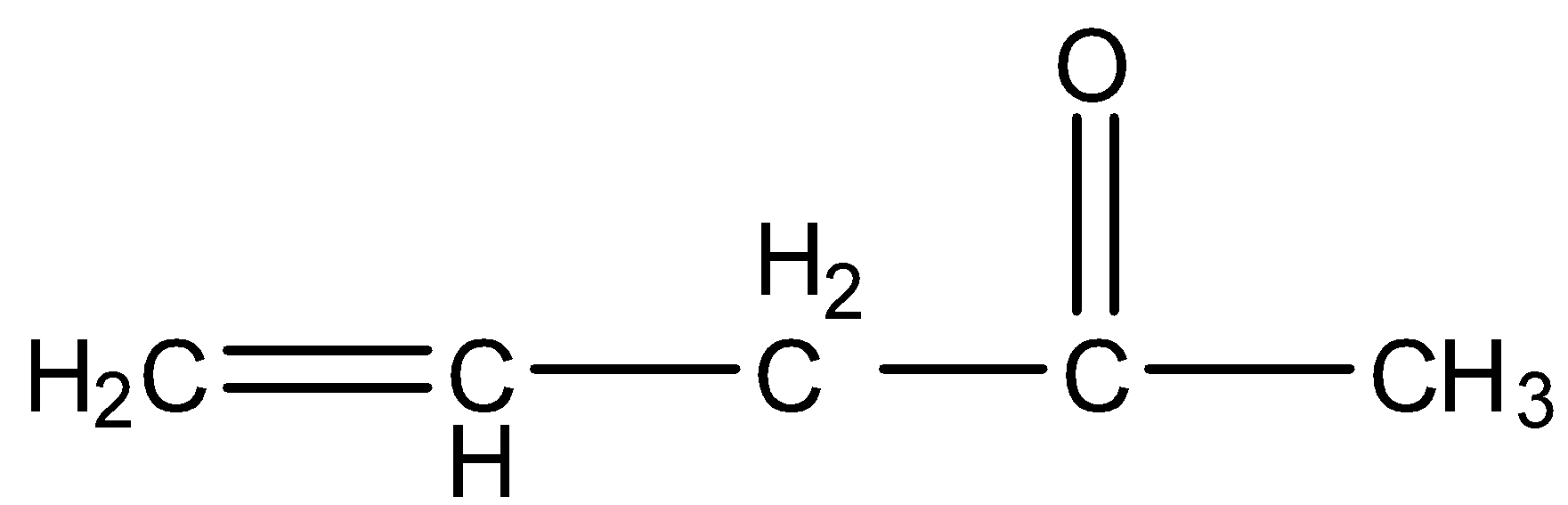
B.

C.
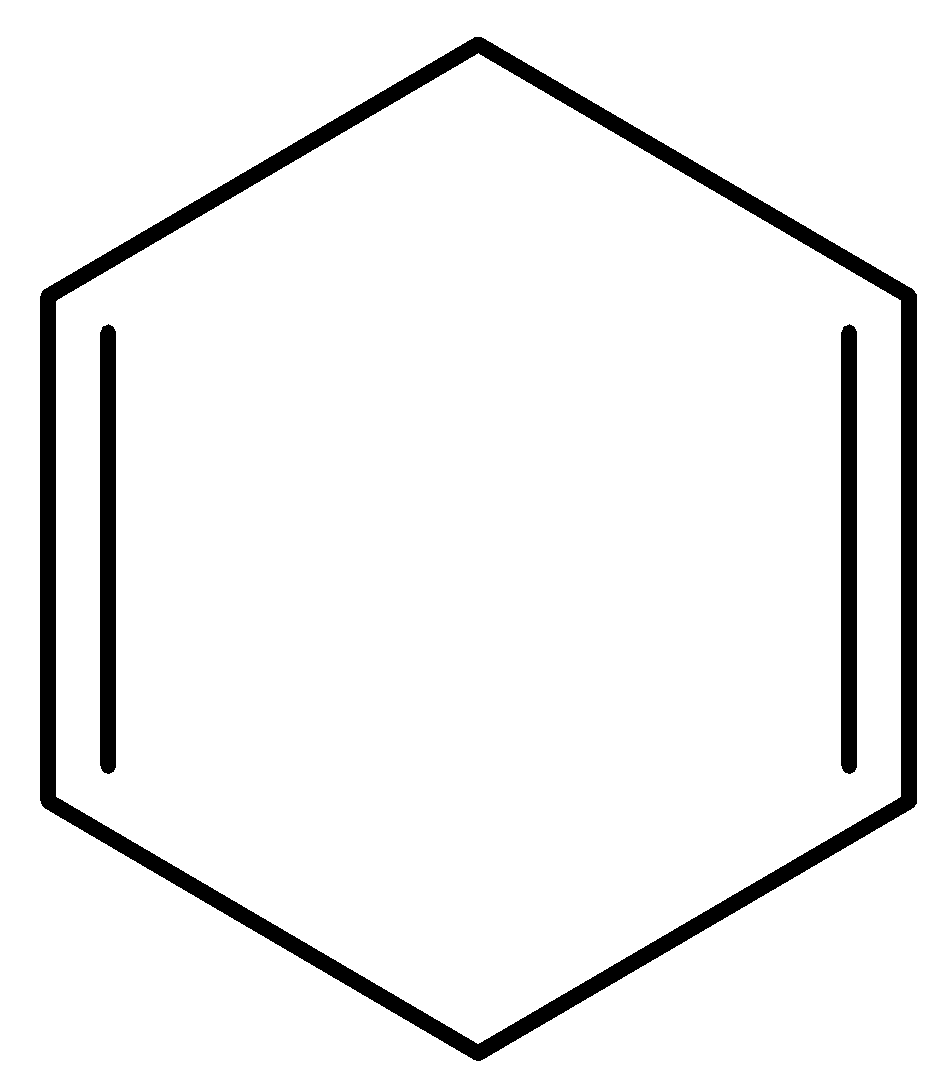
D.
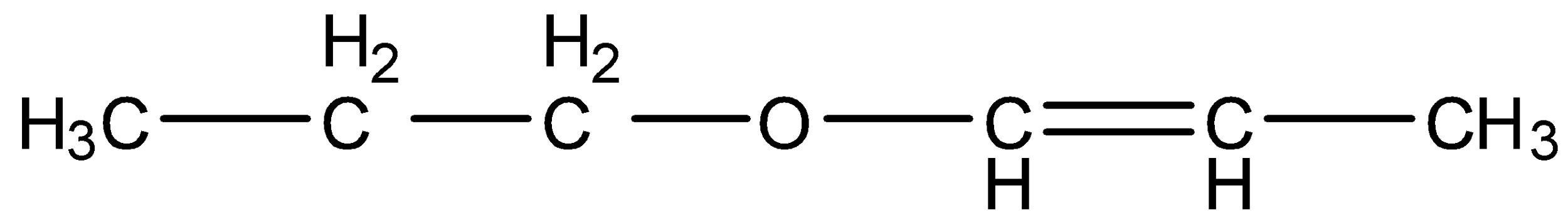




Answer
524.4k+ views
Hint: The electrons which are present in the molecule are always going to move in between the atoms of the atoms then the electrons are called delocalized electrons. The atoms of the compounds should have extra electrons and those electrons are not going to be involved in the permanent bonding.
Complete answer:
- In the question it is asked to find the molecule which contains delocalized electrons among the given options.
- Coming to given options, option A.
.
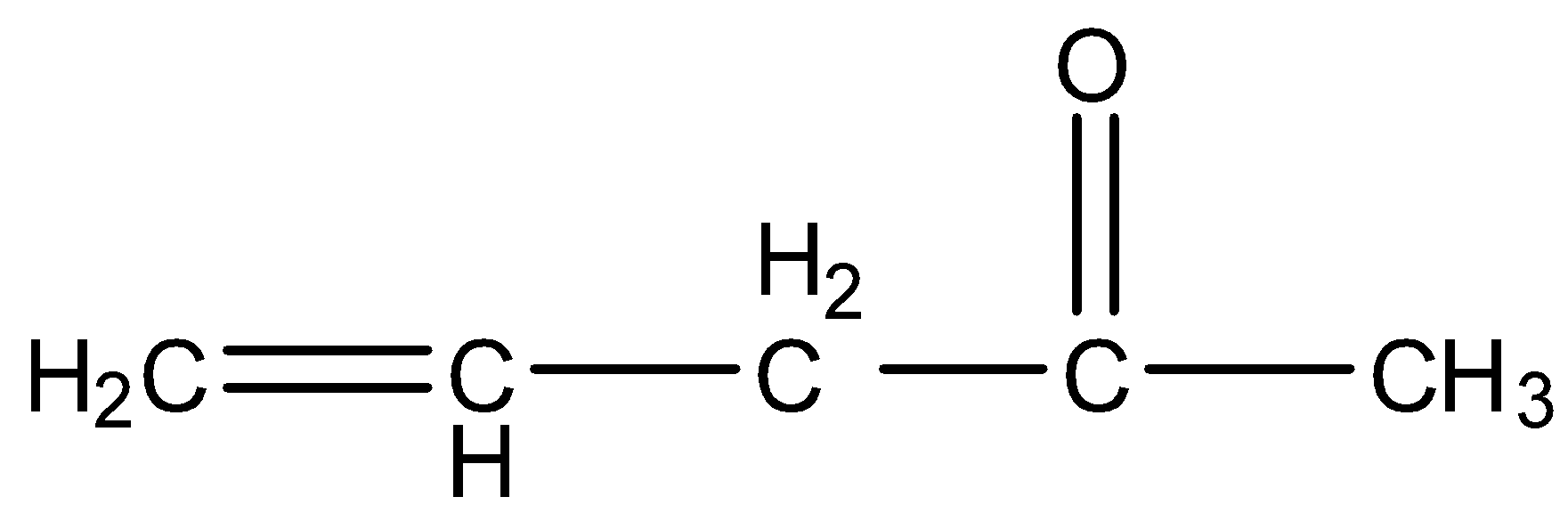
- In the compound A, there are two functional groups alkene and ketone but they not adjacent together, then the compound does not contain a delocalized electron.
- Therefore the option A is incorrect.
- Coming to option B,

- The compound B contains one double bond and one free radical. Again compound B does not contain delocalized electrons because free radical and double bond is not adjacent together.
- Therefore option B is also incorrect.
- Coming to option C,
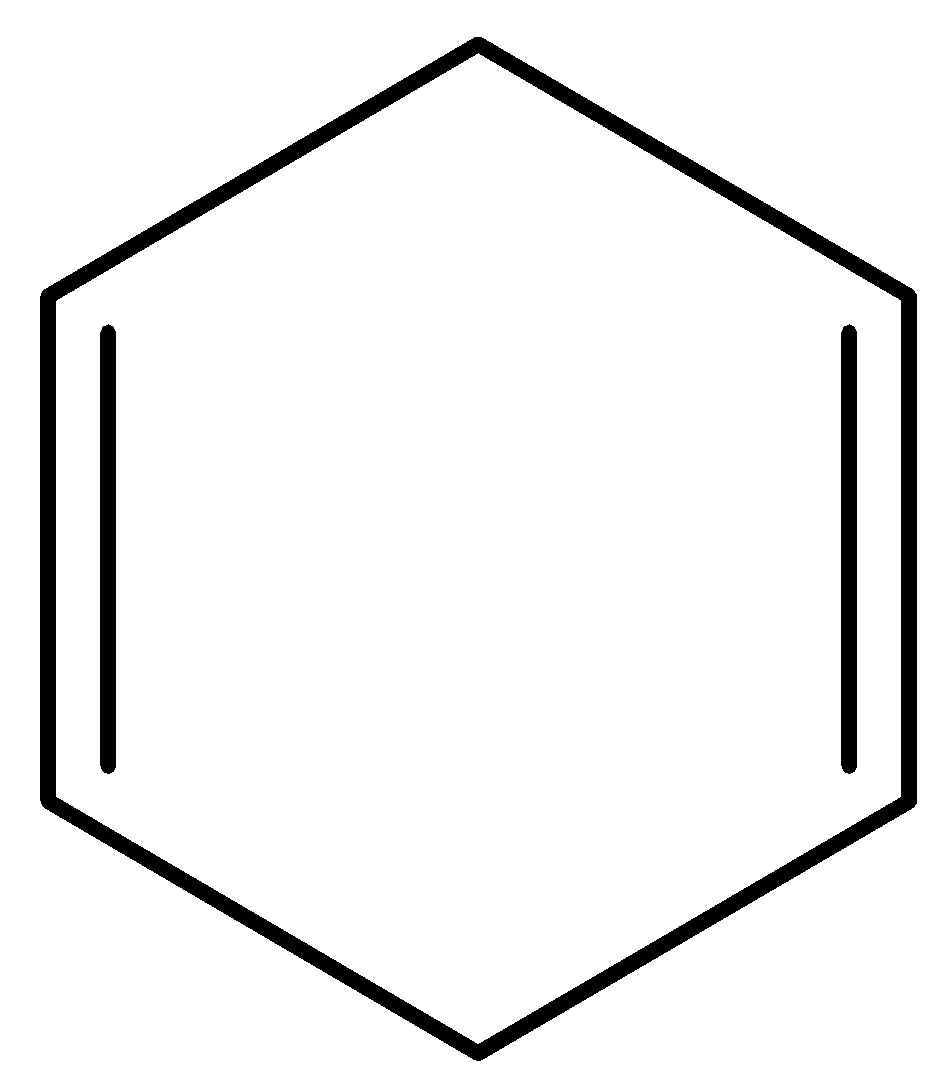
- Compound C contains two double bonds but not adjacent together. Therefore compound C also does not contain delocalized electrons.
- Coming to option D,
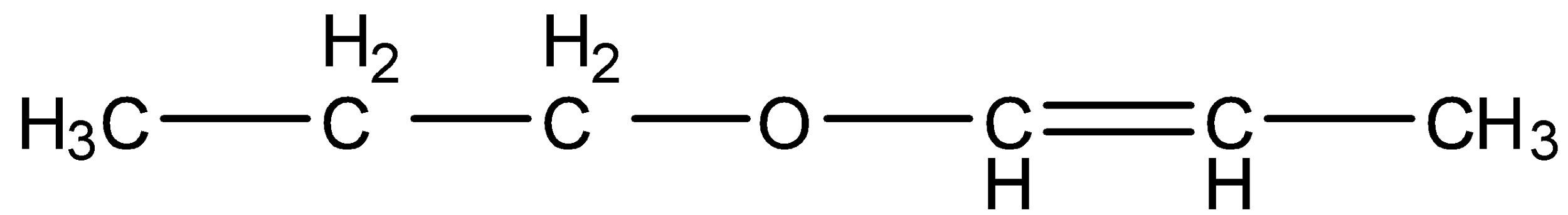 .
.
- Compound D contains two functional groups ether and an alkene. Those two functional groups are adjacent together and we can find the delocalization in it and it is as follows.

- Because of the movement of electrons in the compounds we can see the delocalized electrons clearly.
Therefore option D is the correct answer.
Note:
If the compound contains electrons and they are going to move easily between the different functional groups present in the same molecule then they are called delocalized electrons. The compound should contain nonbonding electrons also too make the availability of delocalized electrons.
Complete answer:
- In the question it is asked to find the molecule which contains delocalized electrons among the given options.
- Coming to given options, option A.
.

- In the compound A, there are two functional groups alkene and ketone but they not adjacent together, then the compound does not contain a delocalized electron.
- Therefore the option A is incorrect.
- Coming to option B,

- The compound B contains one double bond and one free radical. Again compound B does not contain delocalized electrons because free radical and double bond is not adjacent together.
- Therefore option B is also incorrect.
- Coming to option C,

- Compound C contains two double bonds but not adjacent together. Therefore compound C also does not contain delocalized electrons.
- Coming to option D,

- Compound D contains two functional groups ether and an alkene. Those two functional groups are adjacent together and we can find the delocalization in it and it is as follows.

- Because of the movement of electrons in the compounds we can see the delocalized electrons clearly.
Therefore option D is the correct answer.
Note:
If the compound contains electrons and they are going to move easily between the different functional groups present in the same molecule then they are called delocalized electrons. The compound should contain nonbonding electrons also too make the availability of delocalized electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




