
Which of the following compounds will have the highest melting point?
A.Chlorobenzene
B.o-dichlorobenzene
C.m-dichlorobenzene
D.p-dichlorobenzene
Answer
579.9k+ views
Hint: The melting point is usually defined as the point at which the material changes from solid to a liquid. Moreover, melting points may be defined in various ways, each corresponding to a different residual amount of solid or liquid. The main criteria for melting point of such compounds is based on molecular packing.
Complete step by step answer:
Generally, the temperature at which the solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Now, in case of these compounds like o-dichlorobenzene, m-dichlorobenzene and p-dichlorobenzene, the main criteria for determining the melting point is molecular packing. The melting point is directly proportional to the symmetry of the compound. We can say that the symmetry of the compound goes in the same manner as melting point.
So, p-dichlorobenzene is more symmetrical than the o- and m- isomers. Therefore, it fits more closely in the crystal lattice and hence, more energy is required to break the crystal lattice of p-dichlorobenzene. So, it is clear that p-dichlorobenzene will have the highest melting point than the o- and m- isomers. It has a melting point of ${53.5^ \circ }C$ . The three types of isomers are as shown:
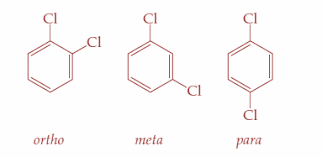
Hence, option D is correct.
Note: p-dichlorobenzene is used as a fumigant insecticide to control clothes moths. It is also found in deodorant blocks made for trash cans and toilets. Most of the people recognize the odor of this compound as the smell of mothballs. Further, acute inhalation or oral exposure to high concentrations may result in liver damage and may cause severe respiratory tract issues.
Complete step by step answer:
Generally, the temperature at which the solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Now, in case of these compounds like o-dichlorobenzene, m-dichlorobenzene and p-dichlorobenzene, the main criteria for determining the melting point is molecular packing. The melting point is directly proportional to the symmetry of the compound. We can say that the symmetry of the compound goes in the same manner as melting point.
So, p-dichlorobenzene is more symmetrical than the o- and m- isomers. Therefore, it fits more closely in the crystal lattice and hence, more energy is required to break the crystal lattice of p-dichlorobenzene. So, it is clear that p-dichlorobenzene will have the highest melting point than the o- and m- isomers. It has a melting point of ${53.5^ \circ }C$ . The three types of isomers are as shown:

Hence, option D is correct.
Note: p-dichlorobenzene is used as a fumigant insecticide to control clothes moths. It is also found in deodorant blocks made for trash cans and toilets. Most of the people recognize the odor of this compound as the smell of mothballs. Further, acute inhalation or oral exposure to high concentrations may result in liver damage and may cause severe respiratory tract issues.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




