
Which of the following compounds will not dissolve in aqueous \[NaOH\]?
A.
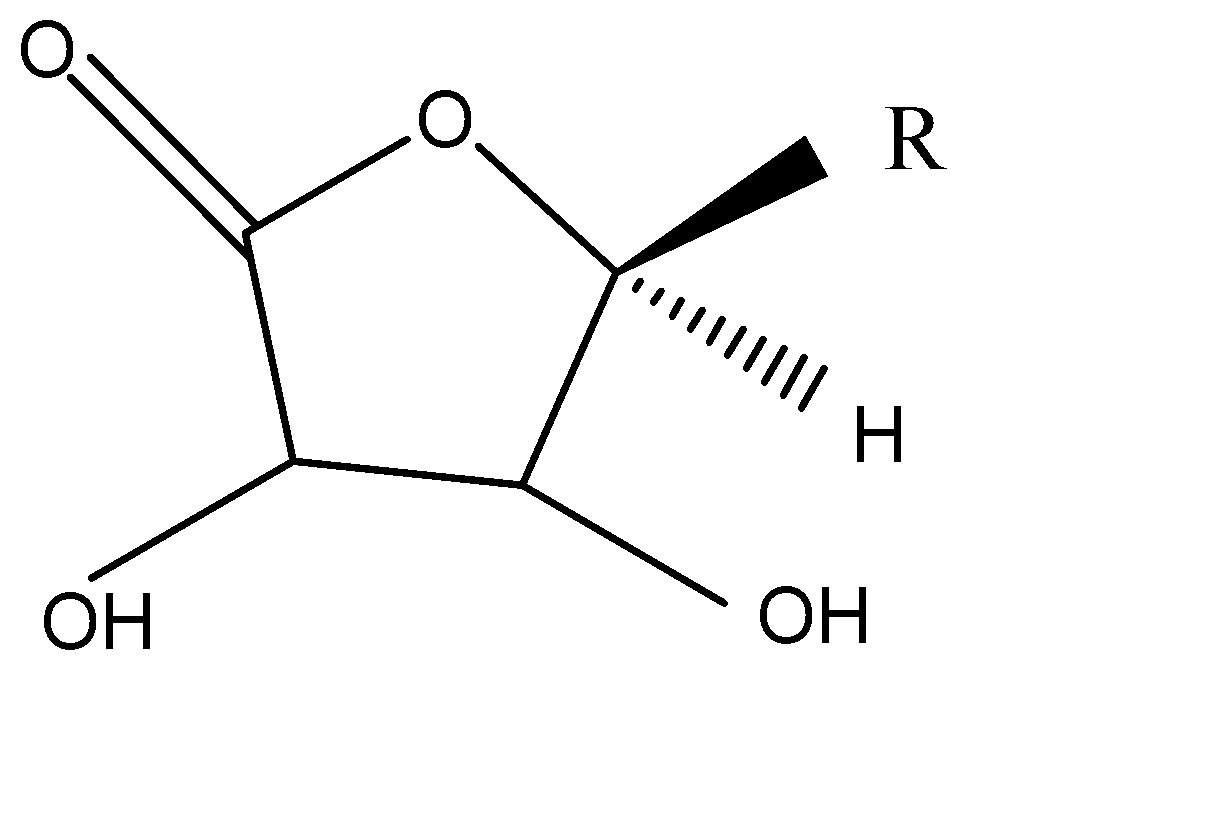
B.

C.
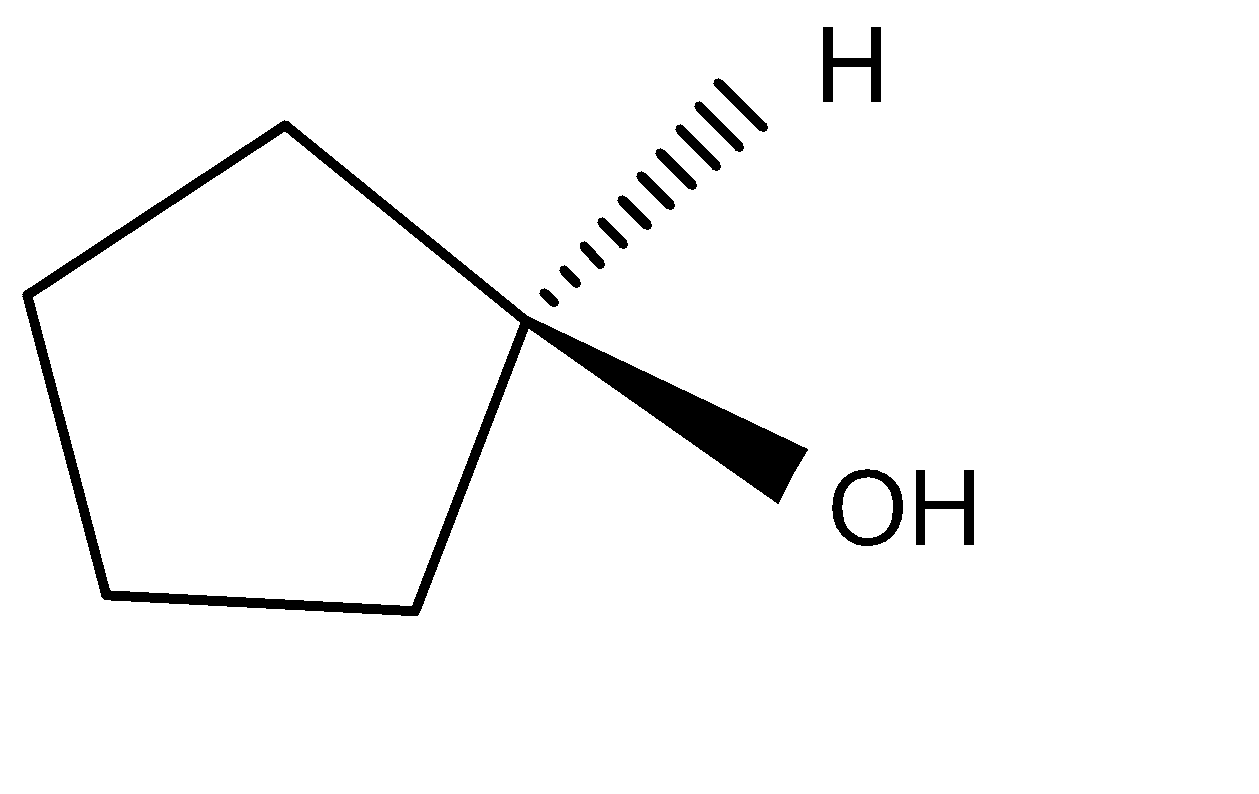
D.
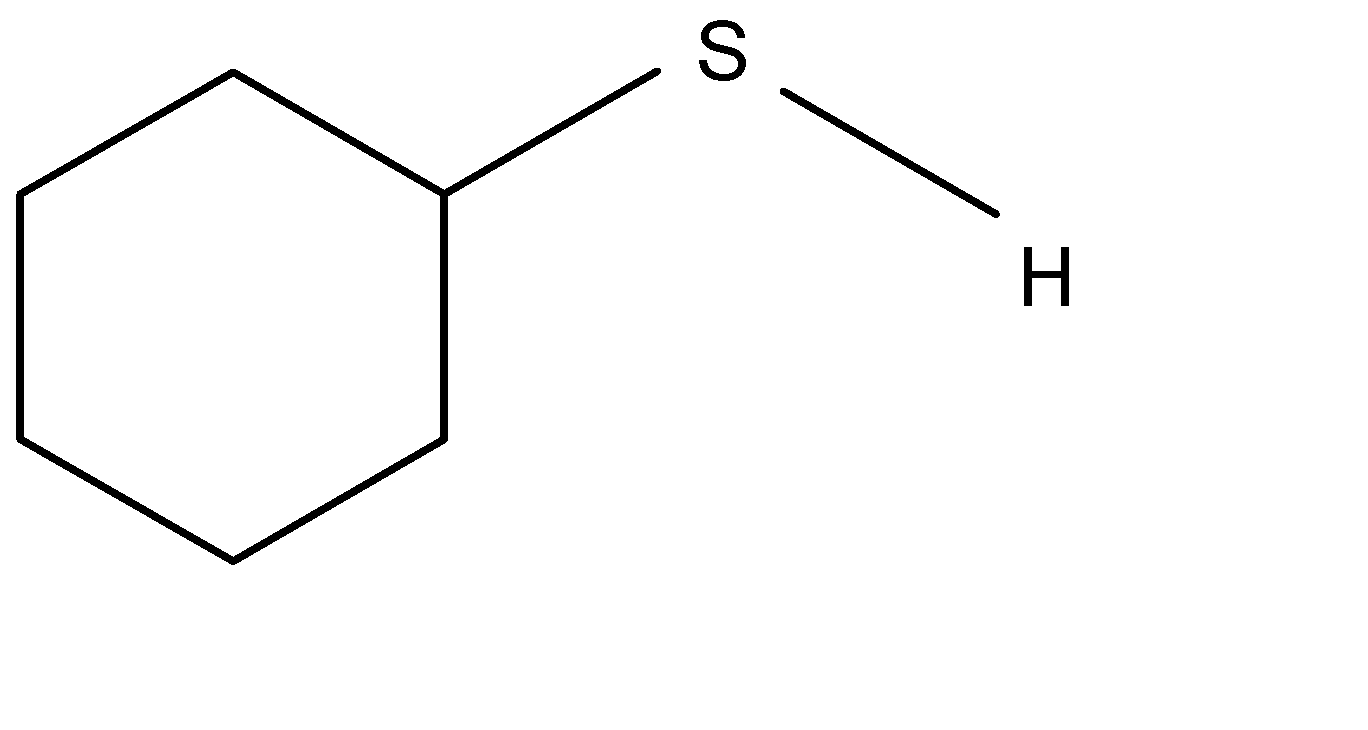




Answer
569.7k+ views
Hint: Determine the solubility of unknowns in water, \[\% \] sodium hydroxide saturated sodium bicarbonate and \[\% \] hydrochloric acid. If the unknown is insoluble in water, but does dissolve in \[5\% \] sodium hydroxide solution then your unknown probably contains an acidic functional group that is deprotonated by the sodium hydroxide producing an ionic compound.
Complete answer:
\[NaOH\] is a strong base. We need strong acids which have acidification and have acidic \[H - \] ions. Here we react to the reaction between strong acid and strong base. Both react and form the conjugate base and contain stability of the compound.
We see option step by step:
In the option A, the reaction forms:
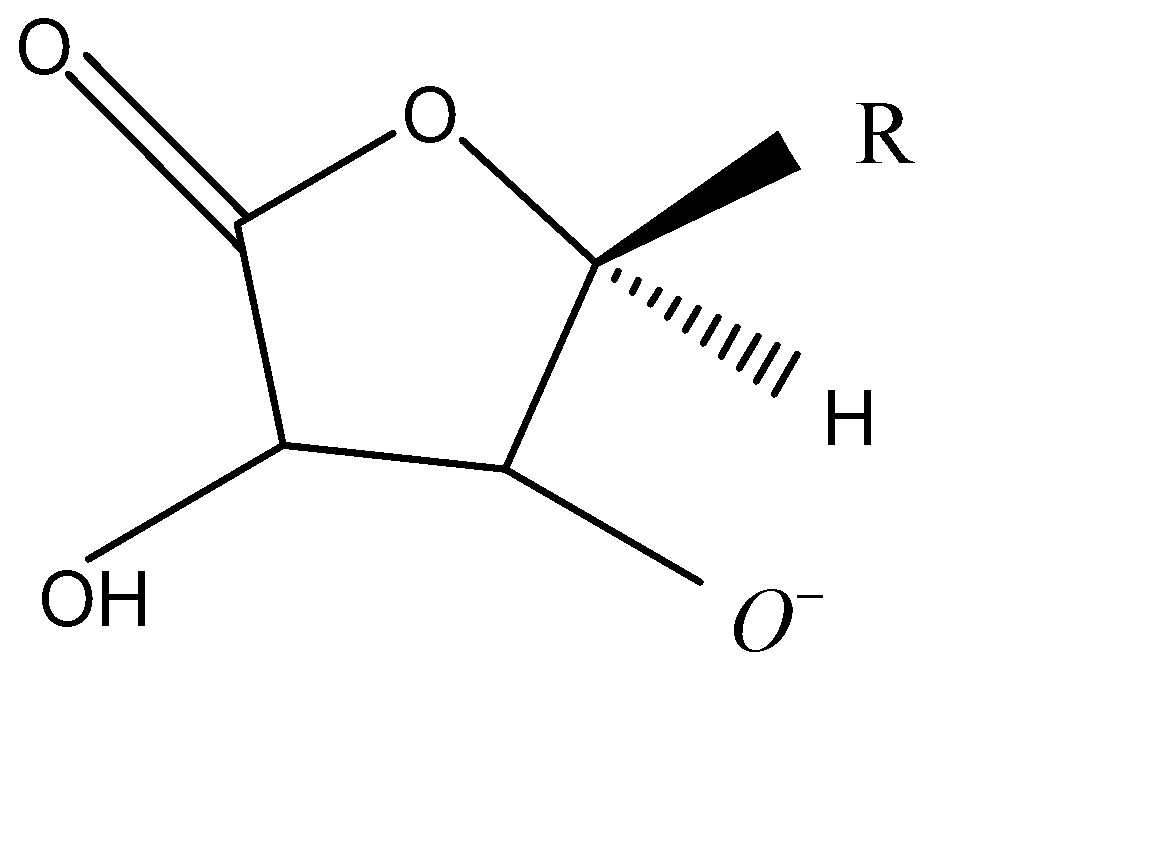
In the option B, the reaction forms:

${O^ - }$
In the option C, the reaction forms:
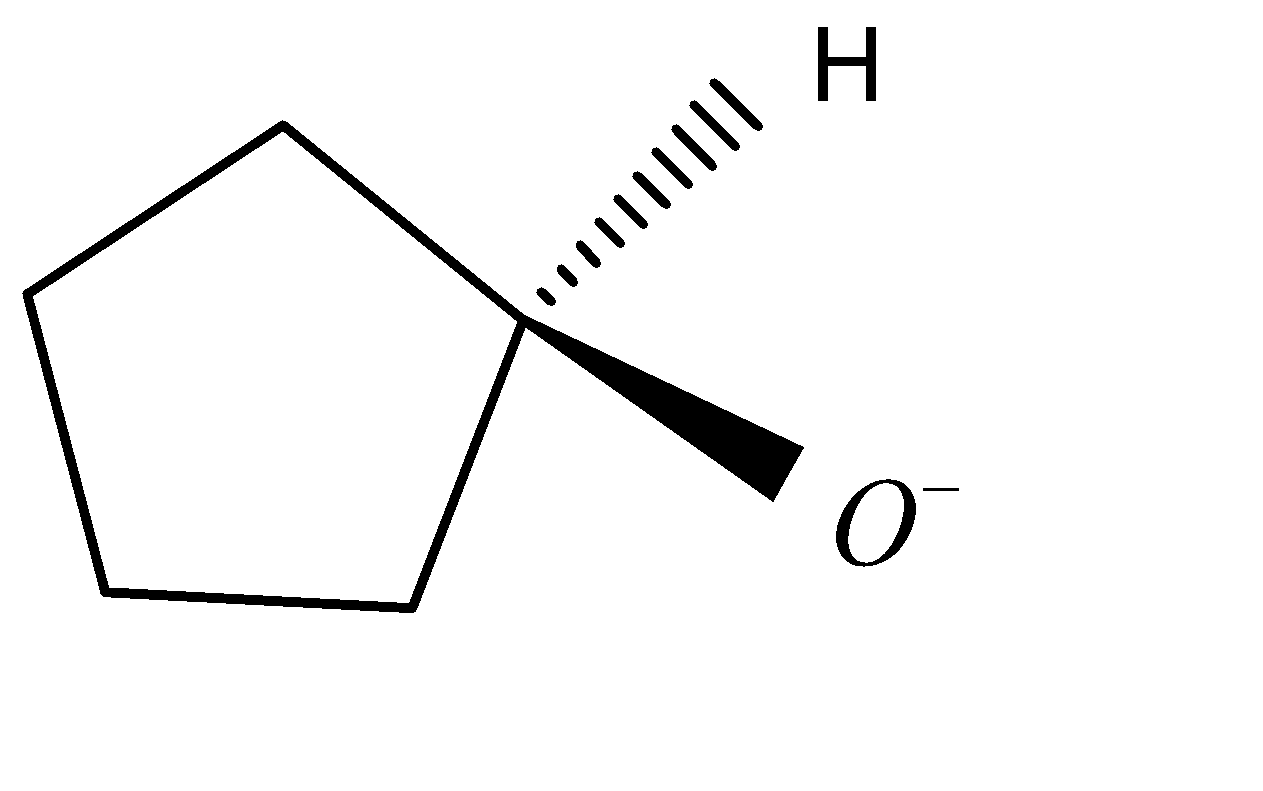
${O^ - }$
In the option D, the reaction forms:

Option (A) contains resonance and it becomes stable and as it is followed in option (B). here option (C) is unstable compound and in option (D) Sulphur has \[-ve\] charge which is easily dispersed then it contains stability.
Hence, option (C) is the correct answer.
Note: The stability of a complex particle in solution is determined by the nature of the central atom and the ligands. The most important characteristics of the central atom, determining the stability of the complex compound, are the degree of oxidation the dimensions, and the electronic structure. In the case of complexes with monatomic ligands, stability is dependent on the same characteristics in the ligand.
Complete answer:
\[NaOH\] is a strong base. We need strong acids which have acidification and have acidic \[H - \] ions. Here we react to the reaction between strong acid and strong base. Both react and form the conjugate base and contain stability of the compound.
We see option step by step:
In the option A, the reaction forms:

In the option B, the reaction forms:

${O^ - }$
In the option C, the reaction forms:

${O^ - }$
In the option D, the reaction forms:

Option (A) contains resonance and it becomes stable and as it is followed in option (B). here option (C) is unstable compound and in option (D) Sulphur has \[-ve\] charge which is easily dispersed then it contains stability.
Hence, option (C) is the correct answer.
Note: The stability of a complex particle in solution is determined by the nature of the central atom and the ligands. The most important characteristics of the central atom, determining the stability of the complex compound, are the degree of oxidation the dimensions, and the electronic structure. In the case of complexes with monatomic ligands, stability is dependent on the same characteristics in the ligand.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




