
Which of the following compounds will not undergo Friedel Crafts reaction with benzene?


Answer
568.8k+ views
Hint:We know that Friedel Crafts reaction usually shows the addition of the halogen group to the alkyl ring. It usually gives two types of reaction one is alkylation, and the second is acylation reaction. They both are preceded by the electrophilic aromatic substitution.
Complete step-by-step answer :It is known to us that the electrophilic substitution reactions are carried out in the presence of catalysts that help in the generation of the electrophile. The reaction of benzene with haloalkanes in the presence of a catalyst, such as \[{\rm{AlC}}{{\rm{l}}_3}\] to give alkyl benzene are regarded as Friedel-Craft alkylation.
From all four given compounds, the option (C) compound generally will not undergo Friedel Crafts reaction with benzene because the compound (C) forms an intermediate carbocation. The carbocation will be dislocated, and the positive charge will be getting more electronegative at the ${\rm{s}}{{\rm{p}}^{\rm{2}}}$ hybridization carbon atom.
The carbonation form by compound C is shown below.
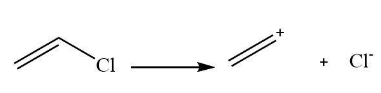
Note:The Friedel craft's reaction is one of the best reactions used for converting the alkyl ring to the halogen attached ring. It is very useful for the synthesis of several chemical compounds. It is also used for the manufacturing of organic compounds in the laboratory. The halogens are chlorine that is Cl, bromine that is Br, and fluorine that is F are usually used in Friedel craft’s reaction.
Complete step-by-step answer :It is known to us that the electrophilic substitution reactions are carried out in the presence of catalysts that help in the generation of the electrophile. The reaction of benzene with haloalkanes in the presence of a catalyst, such as \[{\rm{AlC}}{{\rm{l}}_3}\] to give alkyl benzene are regarded as Friedel-Craft alkylation.
From all four given compounds, the option (C) compound generally will not undergo Friedel Crafts reaction with benzene because the compound (C) forms an intermediate carbocation. The carbocation will be dislocated, and the positive charge will be getting more electronegative at the ${\rm{s}}{{\rm{p}}^{\rm{2}}}$ hybridization carbon atom.
The carbonation form by compound C is shown below.

Note:The Friedel craft's reaction is one of the best reactions used for converting the alkyl ring to the halogen attached ring. It is very useful for the synthesis of several chemical compounds. It is also used for the manufacturing of organic compounds in the laboratory. The halogens are chlorine that is Cl, bromine that is Br, and fluorine that is F are usually used in Friedel craft’s reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




