
Which of the following compounds would yield trialkyl borane shown below when treated with $ B{H_3}/THF $ ?
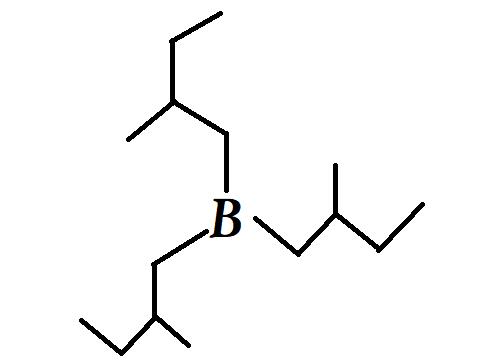
(A) 2-methylbut-1-ene
(B) 2-methylbut-2-ene
(C) 3-methylbut-1-ene
(D) 3-methylbut-1-yne

Answer
517.5k+ views
Hint :In the given question firstly we have to define what exactly is the reaction which takes place under the influence of $ B{H_3}/THF $ and then when are they feasible and goes through with the process. Now we know that the hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol.
Complete Step By Step Answer:
The given question statement asks about the validation of the assertion and the reasoning provided in the problem statement. Here we have to go through both of them and then observe whether both are right and if so then whether they validate each other's occurrences or not in the process.
Firstly we have to give the slight explanation of the Hydroboration oxidation Reaction, which is that In organic chemistry, the hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. In This process the results in the syn addition of the hydrogen and the hydroxyl group where the double bond had been.
We can also say that the Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon.
By this we can say that the right answer would be option A, 2-methylbut-1-yne.
Note :
Use of other oxidants instead of hydrogen peroxide can lead to carbonyl products rather than alcohols from alkenes. N-Methylmorpholine N-oxide with catalytic tetrapropylammonium perruthenate converts the alkylborane into a carbonyl, thus a ketone or aldehyde product depending on what other groups were attached to that carbon in the original alkene.
Complete Step By Step Answer:
The given question statement asks about the validation of the assertion and the reasoning provided in the problem statement. Here we have to go through both of them and then observe whether both are right and if so then whether they validate each other's occurrences or not in the process.
Firstly we have to give the slight explanation of the Hydroboration oxidation Reaction, which is that In organic chemistry, the hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. In This process the results in the syn addition of the hydrogen and the hydroxyl group where the double bond had been.
We can also say that the Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon.
By this we can say that the right answer would be option A, 2-methylbut-1-yne.
Note :
Use of other oxidants instead of hydrogen peroxide can lead to carbonyl products rather than alcohols from alkenes. N-Methylmorpholine N-oxide with catalytic tetrapropylammonium perruthenate converts the alkylborane into a carbonyl, thus a ketone or aldehyde product depending on what other groups were attached to that carbon in the original alkene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




