
Which of the following cycloalkanes has least ring strain?
A.Cyclopropane
B.Cyclobutene
C.Cyclopentane
D.Cyclohexane
Answer
579.6k+ views
Hint: Basically, cycloalkanes are a class of hydrocarbons which have a ring like structure. This ring is formed due to their saturated nature and moreover they have three compounds of alkane present in the structure which helps them in forming a ring.
Complete step by step answer:
The different organic molecules of organic compounds have different properties based upon their structure such as cycloalkanes. These are the classes of hydrocarbons that have a ring like structure. Some common examples of cycloalkanes are cyclopentane, cyclobutene, cyclohexane, cycloheptane etc. Basically, the number of carbon atoms present in the compound decides the structure of the cycloalkane. Some of the structures are as shown:
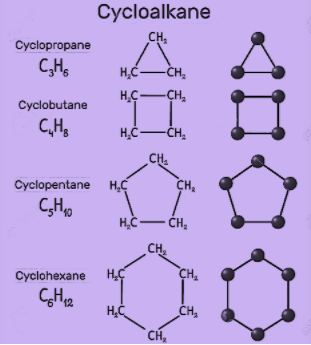
Now, let’s discuss some of the properties of these compounds.
1.These compounds have high melting point and densities
2.These saturated hydrocarbons have boiling point ranging between 10-20K.
3.These are generally insoluble in water. Moreover, the cycloalkanes in liquid form are said to be the good form of solvents for other organic compounds
4.Cyclopropane is considered as the most reactive compound.
Now, among the given options, cyclohexane has the least ring-strain. It has six carbon atoms in the ring. As the number of carbon atoms increases, the ring-strain decreases. The decreasing order of ring strain is 3>4>5>6. The number represents the number of carbon atoms in the ring.
Hence, option D is correct.
Note: Cycloalkanes have various applications. They are used as an organic solvent in the production of drugs. They are utilized in the manufacture of hair products as well as in the food industries. Moreover, some classes of cycloalkanes are used for pigmentation purposes and also used as fragrances in the perfume manufacturing sector.
Complete step by step answer:
The different organic molecules of organic compounds have different properties based upon their structure such as cycloalkanes. These are the classes of hydrocarbons that have a ring like structure. Some common examples of cycloalkanes are cyclopentane, cyclobutene, cyclohexane, cycloheptane etc. Basically, the number of carbon atoms present in the compound decides the structure of the cycloalkane. Some of the structures are as shown:

Now, let’s discuss some of the properties of these compounds.
1.These compounds have high melting point and densities
2.These saturated hydrocarbons have boiling point ranging between 10-20K.
3.These are generally insoluble in water. Moreover, the cycloalkanes in liquid form are said to be the good form of solvents for other organic compounds
4.Cyclopropane is considered as the most reactive compound.
Now, among the given options, cyclohexane has the least ring-strain. It has six carbon atoms in the ring. As the number of carbon atoms increases, the ring-strain decreases. The decreasing order of ring strain is 3>4>5>6. The number represents the number of carbon atoms in the ring.
Hence, option D is correct.
Note: Cycloalkanes have various applications. They are used as an organic solvent in the production of drugs. They are utilized in the manufacture of hair products as well as in the food industries. Moreover, some classes of cycloalkanes are used for pigmentation purposes and also used as fragrances in the perfume manufacturing sector.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




