
Which of the following does not undergo Cannizzaro reaction?
A. Benzaldehyde
B. 2-methylpropanal
C. p-methoxy benzaldehyde
D. 2,2-dimethylpropanal
Answer
514.2k+ views
Hint :An organic reaction in which two non-enolizable aldehydes reacts together in the presence of sodium hydroxide to undergo a disproportionation reaction is known as a Cannizzaro reaction. A carboxylic acid and primary alcohol are formed as the products after the reaction.
Complete Step By Step Answer:
Basically, Cannizzaro reaction is a type of nucleophilic acyl substitution reaction and the primary criteria for a compound to undergo this reaction is it must not have any alpha hydrogen i.e., the number of hydrogen atoms at the alpha carbon (first carbon atom which is attached to the carbonyl group) must be equal to zero.
Therefore, among given options the compounds which undergo the Cannizzaro reaction are as follows:
Benzaldehyde-
The structure of benzaldehyde is as follows:
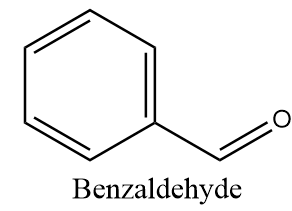
The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is present in the benzene ring due to which it does not consist of any hydrogen atom. Thus, the compound has no alpha hydrogen and can undergo a Cannizzaro reaction.
2-methylpropanal:
The structure of 2-methylpropanal is as follows:
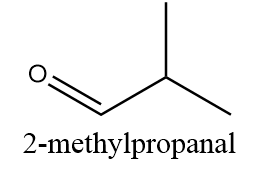
The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is connected to two methyl groups and one hydrogen atom as follows:
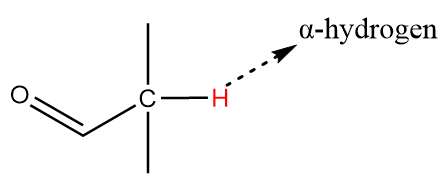
Thus, the compound has one alpha hydrogen and cannot undergo a Cannizzaro reaction.
p-methoxy benzaldehyde:
The structure of p-methoxy benzaldehyde is as follows:
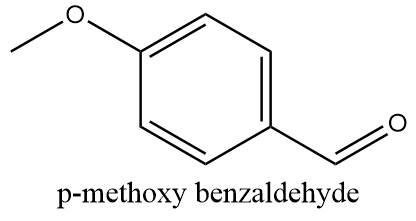
The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is present in the benzene ring due to which it does not consist of any hydrogen atom. Thus, the compound has no alpha hydrogen and can undergo a Cannizzaro reaction.
2,2-dimethylpropanal:
The structure of 2,2-dimethylpropanal is as follows:
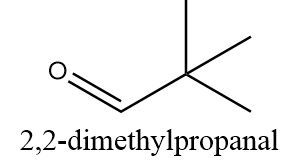
The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is connected to three methyl groups and no hydrogen atom is bonded to it. Thus, the compound has no alpha hydrogen and can undergo a Cannizzaro reaction.
Hence, the compound which cannot undergo a Cannizzaro reaction is 2-methylpropanal.
So, option (B) is the correct answer.
Note :
It is important to note that if two different non-enolizable aldehydes react together in the basic medium, then one of the aldehydes is oxidized while the other yields a reduction product. It is a special case of Cannizzaro reaction known as cross Cannizzaro reaction. In the reaction, less bulky molecules are oxidized whereas the bulky molecules are easily attacked by base so are reduced after the reaction.
Complete Step By Step Answer:
Basically, Cannizzaro reaction is a type of nucleophilic acyl substitution reaction and the primary criteria for a compound to undergo this reaction is it must not have any alpha hydrogen i.e., the number of hydrogen atoms at the alpha carbon (first carbon atom which is attached to the carbonyl group) must be equal to zero.
Therefore, among given options the compounds which undergo the Cannizzaro reaction are as follows:
Benzaldehyde-
The structure of benzaldehyde is as follows:

The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is present in the benzene ring due to which it does not consist of any hydrogen atom. Thus, the compound has no alpha hydrogen and can undergo a Cannizzaro reaction.
2-methylpropanal:
The structure of 2-methylpropanal is as follows:

The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is connected to two methyl groups and one hydrogen atom as follows:

Thus, the compound has one alpha hydrogen and cannot undergo a Cannizzaro reaction.
p-methoxy benzaldehyde:
The structure of p-methoxy benzaldehyde is as follows:

The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is present in the benzene ring due to which it does not consist of any hydrogen atom. Thus, the compound has no alpha hydrogen and can undergo a Cannizzaro reaction.
2,2-dimethylpropanal:
The structure of 2,2-dimethylpropanal is as follows:

The alpha carbon i.e., the carbon atom adjacent to the carbonyl group is connected to three methyl groups and no hydrogen atom is bonded to it. Thus, the compound has no alpha hydrogen and can undergo a Cannizzaro reaction.
Hence, the compound which cannot undergo a Cannizzaro reaction is 2-methylpropanal.
So, option (B) is the correct answer.
Note :
It is important to note that if two different non-enolizable aldehydes react together in the basic medium, then one of the aldehydes is oxidized while the other yields a reduction product. It is a special case of Cannizzaro reaction known as cross Cannizzaro reaction. In the reaction, less bulky molecules are oxidized whereas the bulky molecules are easily attacked by base so are reduced after the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




