
Which of the following does NOT undergo haloform reaction?
A. \[C{{H}_{3}}-CH(OH)-C{{H}_{3}}\]
B. \[C{{H}_{3}}-CH(O)-C{{H}_{3}}\]
C. \[{{C}_{2}}{{H}_{5}}-CH(OH)-{{C}_{2}}{{H}_{5}}\]
D. \[C{{H}_{3}}-C(O)-{{C}_{2}}{{H}_{5}}\]
Answer
597.3k+ views
Hint: As the name suggests, haloform reaction is a type of reaction which will result in the form of haloforms such as chloroform, bromoform and iodoform. Compounds with the alpha methyl group gives this test.
Complete step by step answer:
Haloform test is a distinction test. This test is given by aldehydes (mainly) and ketones that contain the alpha-methyl group.
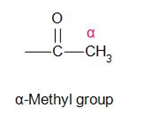
Let us draw all the compounds given in the question and check it for the ‘alpha-methyl group’.
Option (a)
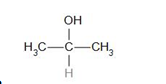
The structure of the compound given in option (a) is a secondary alcohol. It does not contain the alpha-methyl group.
Therefore, it does not give a haloform test.
Option (b)
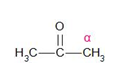
The structure of the compound given in option (b) is a secondary ketone. It contains the alpha-methyl group.
Therefore, it gives the haloform test.
Option (c)
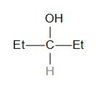
The structure of the compound given in option (c) is a secondary alcohol. It does not contain the alpha-methyl group.
Therefore, it does not give a haloform test.
Option (d)
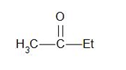
The structure of the compound given in option (d) is a secondary ketone. It does not contain the alpha-methyl group.
Therefore, it does not give a haloform test.
Therefore, the answer is – option (b).
Additional Information:
Chloroform is a colourless liquid. Bromoform is a colourless liquid with a sweet smell. Iodoform forms a pale-yellow precipitate with funky smell.
Note: The reaction in haloform takes place through the following reaction –
\[R-C(O)-C{{H}_{3}}+3{{X}_{2}}+4NaOH\to R{{X}_{3}}+C{{H}_{3}}COONa+3NaX+4{{H}_{2}}O\]
Where, X – halogen (Cl, Br, I).
Complete step by step answer:
Haloform test is a distinction test. This test is given by aldehydes (mainly) and ketones that contain the alpha-methyl group.

Let us draw all the compounds given in the question and check it for the ‘alpha-methyl group’.
Option (a)

The structure of the compound given in option (a) is a secondary alcohol. It does not contain the alpha-methyl group.
Therefore, it does not give a haloform test.
Option (b)

The structure of the compound given in option (b) is a secondary ketone. It contains the alpha-methyl group.
Therefore, it gives the haloform test.
Option (c)

The structure of the compound given in option (c) is a secondary alcohol. It does not contain the alpha-methyl group.
Therefore, it does not give a haloform test.
Option (d)
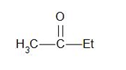
The structure of the compound given in option (d) is a secondary ketone. It does not contain the alpha-methyl group.
Therefore, it does not give a haloform test.
Therefore, the answer is – option (b).
Additional Information:
Chloroform is a colourless liquid. Bromoform is a colourless liquid with a sweet smell. Iodoform forms a pale-yellow precipitate with funky smell.
Note: The reaction in haloform takes place through the following reaction –
\[R-C(O)-C{{H}_{3}}+3{{X}_{2}}+4NaOH\to R{{X}_{3}}+C{{H}_{3}}COONa+3NaX+4{{H}_{2}}O\]
Where, X – halogen (Cl, Br, I).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




