
Which of the following electronic configurations have the highest exchange energy?
(A)
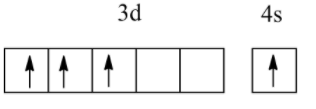
(B)
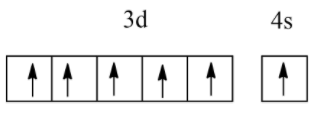
(C)
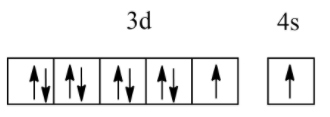
(D)
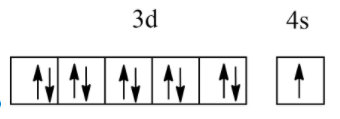




Answer
578.4k+ views
Hint: Different atoms in which the filling of electrons into the orbitals takes place according to the Aufbau principle which is based on Pauli’s exclusion principle and Hund’s rule of maximum multiplicity. The filling of electrons into these orbitals is based on mainly the relative energies of orbitals.
Complete step by step solution:
Aufbau Principle:
This principle discussed building up of orbitals which means filling up of orbitals with electrons. According to this principle, “in the ground state of atoms, the orbitals fill in the order of their increasing energies” which means the electrons first occupy the lowest energy available orbital and then enter into higher energy orbitals after filling lower energy orbitals.
The energies of the orbitals increasing order in which the electrons are filled in the orbitals as follows,
1s<2s<2p<3s<3p<4s<3d<4p<<5s<4d<5p<4f<5d<6p<7s….
Pauli Exclusion Principle:
This exclusion principle discussed the number of electrons to be filled in various orbitals is restricted. According to this principle, “no two electrons in an atom have the same set of four quantum numbers” which means the maximum number of electrons in the shell depends on the principal quantum number equal to $2{{n}^{2}}$
Hund’s rule of maximum multiplicity:
This rule discussed with the filling of electrons into the orbitals belongs to the same subshell which means orbitals with equal energy called degenerate orbitals.
This rule states that “pairing of electrons in the orbitals belongs to the subshell does not take place until each orbital belongs to that subshell has one electron each.”
Hence, higher in the unpaired electrons having higher exchange energy.
So, among the above, all electronic configurations, option B has the highest exchange energy due to the maximum number of unpaired electrons.
Note: A quantitatively large number of orbitals can be distinguished by their shape, size, and orientation. A small size orbital means more chance of finding the electron near the nucleus. Atomic orbitals are distinguished by the quantum numbers which are three quantum numbers labeled as n, l, and m.
Complete step by step solution:
Aufbau Principle:
This principle discussed building up of orbitals which means filling up of orbitals with electrons. According to this principle, “in the ground state of atoms, the orbitals fill in the order of their increasing energies” which means the electrons first occupy the lowest energy available orbital and then enter into higher energy orbitals after filling lower energy orbitals.
The energies of the orbitals increasing order in which the electrons are filled in the orbitals as follows,
1s<2s<2p<3s<3p<4s<3d<4p<<5s<4d<5p<4f<5d<6p<7s….
Pauli Exclusion Principle:
This exclusion principle discussed the number of electrons to be filled in various orbitals is restricted. According to this principle, “no two electrons in an atom have the same set of four quantum numbers” which means the maximum number of electrons in the shell depends on the principal quantum number equal to $2{{n}^{2}}$
Hund’s rule of maximum multiplicity:
This rule discussed with the filling of electrons into the orbitals belongs to the same subshell which means orbitals with equal energy called degenerate orbitals.
This rule states that “pairing of electrons in the orbitals belongs to the subshell does not take place until each orbital belongs to that subshell has one electron each.”
Hence, higher in the unpaired electrons having higher exchange energy.
So, among the above, all electronic configurations, option B has the highest exchange energy due to the maximum number of unpaired electrons.
Note: A quantitatively large number of orbitals can be distinguished by their shape, size, and orientation. A small size orbital means more chance of finding the electron near the nucleus. Atomic orbitals are distinguished by the quantum numbers which are three quantum numbers labeled as n, l, and m.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




