
Which of the following graphs indicates chemical isobar?
A)

B)
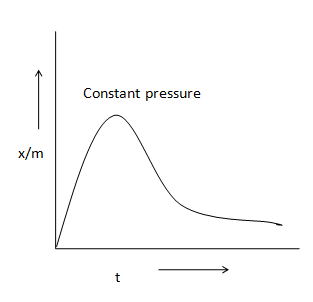
C)
D)





Answer
561.6k+ views
Hint: We have to remember that the isobars are iotas (nuclides) of various synthetic components that have similar numbers of nucleons. Correspondingly, isobars contrast in nuclear number (or number of protons) however have a similar mass number. While the cores of these nuclides all contain 40 nucleons, they contain differing quantities of protons and neutrons.
Complete step by step answer:
We must remember that the chemisorption is a sort of adsorption which includes a compound response between the surface and the adsorbate. New synthetic securities are produced at the adsorbent surface. Models incorporate perceptible marvels that can be extremely self-evident, similar to consumption, and subtler impacts related with heterogeneous catalysis, where the impetus and reactants are in various stages. The solid communication between the adsorbate and the substrate surface makes new kinds of electronic bonds.
Conversely with Chemisorption is physisorption, which leaves the compound types of the adsorbate and surface flawless. Because of particularity, the idea of chemisorption can extraordinarily vary, contingent upon the substance personality and the surface auxiliary properties. The connection between the adsorbate and adsorbent in chemisorption is either ionic or covalent.
We need to remember that the chemisorption requires high initiation energy, so it is alluded to as actuated adsorption.
In chemisorptions adsorption isobar first increments and afterward diminishes with increment in temperature. At the point when adsorption isobar is plotted, the chart first increments and afterward diminishes with temperature.
Therefore, the option (B) is correct.
Note:
Now we can discuss the employments of Isobars as,
-Atomic reactors use isobars of uranium.
-For the therapy of disease, isobars of cobalt are utilized.
-Isobars of iodine are utilized in the treatment of goiter.
Complete step by step answer:
We must remember that the chemisorption is a sort of adsorption which includes a compound response between the surface and the adsorbate. New synthetic securities are produced at the adsorbent surface. Models incorporate perceptible marvels that can be extremely self-evident, similar to consumption, and subtler impacts related with heterogeneous catalysis, where the impetus and reactants are in various stages. The solid communication between the adsorbate and the substrate surface makes new kinds of electronic bonds.
Conversely with Chemisorption is physisorption, which leaves the compound types of the adsorbate and surface flawless. Because of particularity, the idea of chemisorption can extraordinarily vary, contingent upon the substance personality and the surface auxiliary properties. The connection between the adsorbate and adsorbent in chemisorption is either ionic or covalent.
We need to remember that the chemisorption requires high initiation energy, so it is alluded to as actuated adsorption.
In chemisorptions adsorption isobar first increments and afterward diminishes with increment in temperature. At the point when adsorption isobar is plotted, the chart first increments and afterward diminishes with temperature.
Therefore, the option (B) is correct.
Note:
Now we can discuss the employments of Isobars as,
-Atomic reactors use isobars of uranium.
-For the therapy of disease, isobars of cobalt are utilized.
-Isobars of iodine are utilized in the treatment of goiter.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




